Microwave-Assisted Hydrothermal Synthesis of Zn2SnO4 Nanostructures for Photocatalytic Dye Degradation †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
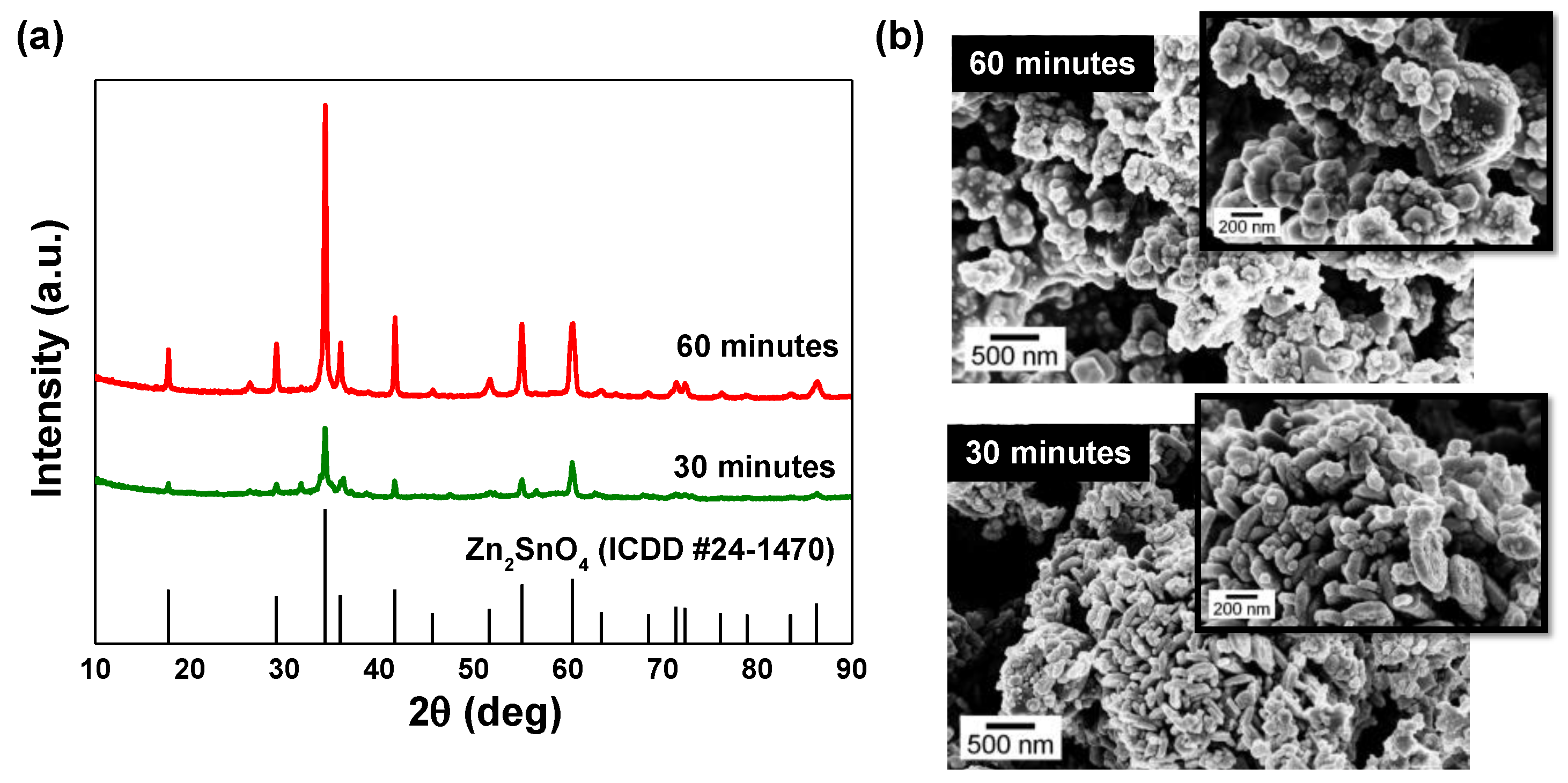
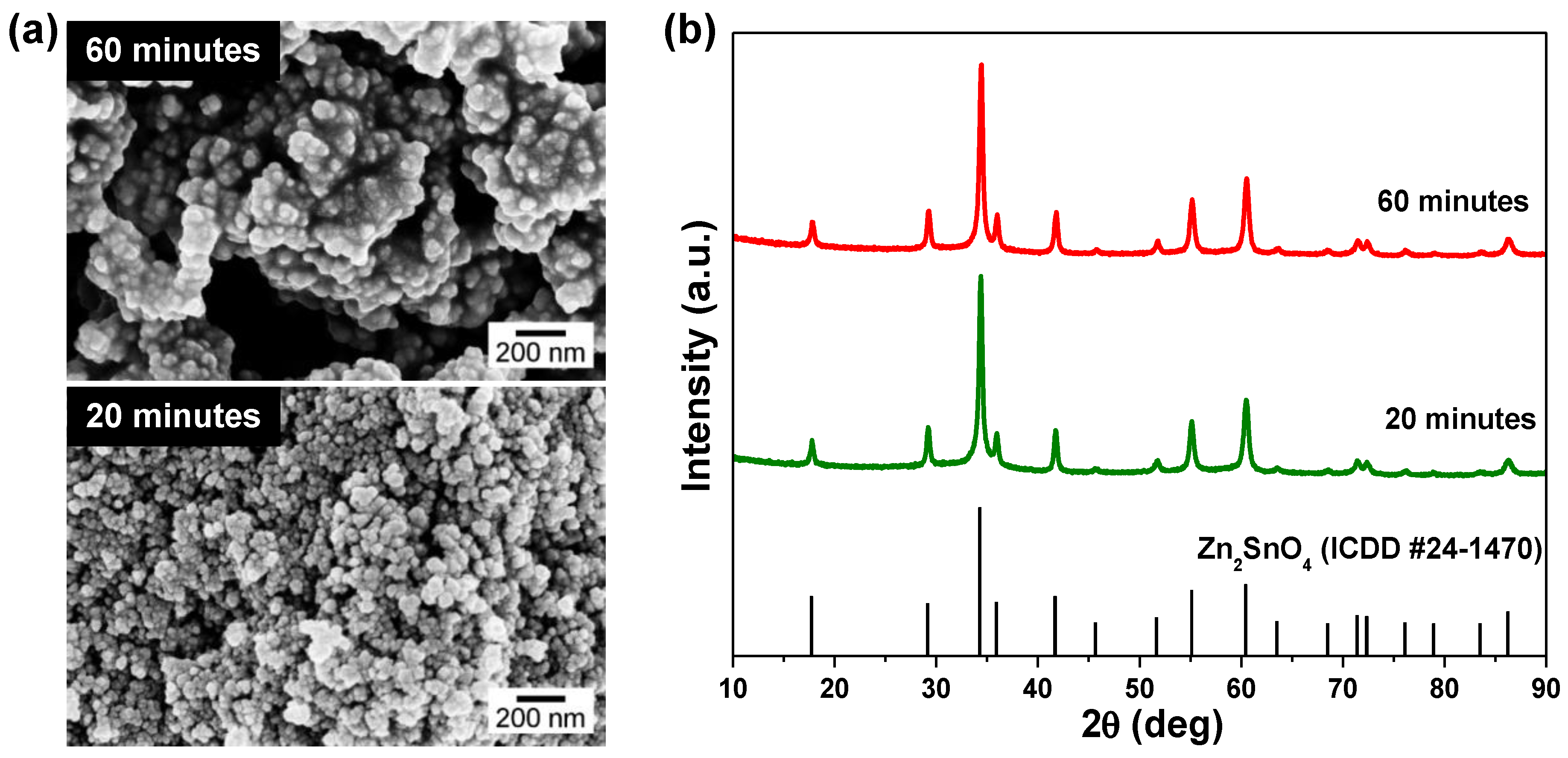
3.1. Microwave-Assisted Synthesis of Zn2SnO4 Nanostructures
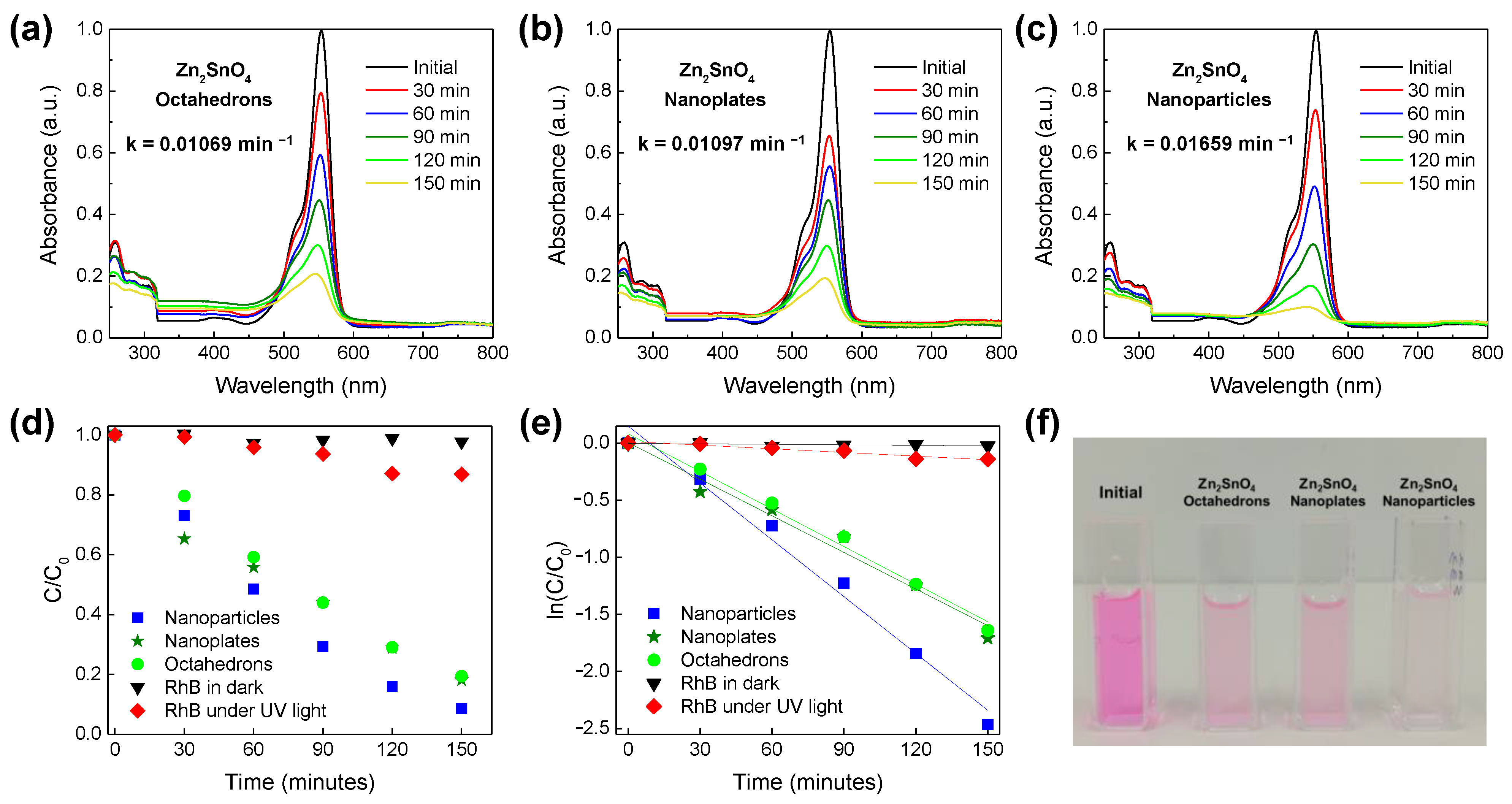
3.2. Photocatalytic Activity of Zn2SnO4 Nanostructures
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baruah, S.; Dutta, J. Zinc stannate nanostructures: Hydrothermal synthesis. Sci. Technol. Adv. Mater. 2011, 12. [Google Scholar] [CrossRef]
- Sun, S.; Liang, S. Morphological zinc stannate: Synthesis, fundamental properties and applications. J. Mater. Chem. A 2017, 5, 20534–20560. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, X.; Zhao, D.; Niu, Z.; Zeng, Q.; Li, J.; Cai, L.; Wang, Y.; Zhou, W.; Gao, M.; et al. High performance bipolar resistive switching memory devices based on Zn2SnO4 nanowires. Nanoscale 2012, 4, 2571–2574. [Google Scholar] [CrossRef]
- Lim, T.; Kim, H.; Meyyappan, M.; Ju, S. Photostable Zn2SnO4 Nanowire Transistors for Transparent Displays. ACS Nano 2012, 6, 4912–4920. [Google Scholar] [CrossRef]
- Rovisco, A.; dos Santos, A.; Cramer, T.; Martins, J.; Branquinho, R.; Águas, H.; Fraboni, B.; Fortunato, E.; Martins, R.; Igreja, R.; et al. Piezoelectricity Enhancement of Nanogenerators Based on PDMS and ZnSnO 3 Nanowires through Microstructuration. ACS Appl. Mater. Interfaces 2020, 12, 18421–18430. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, Q.; Shen, G.; Wang, R.; Shen, G. Ternary oxide nanostructured materials for supercapacitors: A review. J. Mater. Chem. A Mater. Energy Sustain. 2015, 3, 10158–10173. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Gierlotka, S.; Lojkowski, W. Effect of Microwave Radiation Power on the Size of Aggregates of ZnO NPs Prepared Using Microwave Solvothermal Synthesis. Nanomaterials 2018, 8, 343. [Google Scholar] [CrossRef]
- Lehnen, T.; Zopes, D.; Mathur, S. Phase-selective microwave synthesis and inkjet printing applications of Zn2SnO4 (ZTO) quantum dots. J. Mater. Chem. 2012, 22, 17732. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef]
- Pimentel, A.; Ferreira, S.; Nunes, D.; Calmeiro, T.; Martins, R.; Fortunato, E. Microwave Synthesized ZnO Nanorod Arrays for UV Sensors: A Seed Layer Annealing Temperature Study. Materials 2016, 9, 299. [Google Scholar] [CrossRef]
- Pimentel, A.; Nunes, D.; Duarte, P.; Rodrigues, J.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Synthesis of Long ZnO Nanorods under Microwave Irradiation or Conventional Heating. J. Phys. Chem. C 2014, 118, 14629–14639. [Google Scholar] [CrossRef]
- Pimentel, A.; Rodrigues, J.; Duarte, P.; Nunes, D.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Effect of solvents on ZnO nanostructures synthesized by solvothermal method assisted by microwave radiation: A photocatalytic study. J. Mater. Sci. 2015, 50, 5777–5787. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Pinto, J.V.; Calmeiro, T.R.; Nandy, S.; Barquinha, P.; Pereira, L.; Carvalho, P.A.; Fortunato, E.; Martins, R. Photocatalytic behavior of TiO2 films synthesized by microwave irradiation. Catal. Today 2016, 278, 262–270. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Barquinha, P.; Carvalho, P.A.; Fortunato, E.; Martins, R. Cu2O polyhedral nanowires produced by microwave irradiation. J. Mater. Chem. C 2014, 2, 6097. [Google Scholar] [CrossRef]
- Xiao, L.; Shen, H.; Von Hagen, R.; Pan, J.; Belkoura, L.; Mathur, S. Microwave assisted fast and facile synthesis of SnO2 quantum dots and their printing applications. Chem. Commun. 2010, 46, 6509–6511. [Google Scholar] [CrossRef] [PubMed]
- Nehru, L.C.; Sanjeeviraja, C. Controllable growth of Zn2SnO4 nanostructures by urea assisted microwave-assisted solution combustion process. J. Ceram. Process. Res. 2013, 14, 606–609. [Google Scholar]
- Reyes, O.; Pal, M.; Escorcia-García, J.; Sánchez-Albores, R.; Sebastian, P.J. Microwave-assisted chemical synthesis of Zn2SnO4 nanoparticles. Mater. Sci. Semicond. Process. 2020, 108, 104878. [Google Scholar] [CrossRef]
- Jain, S.; Shah, A.P.; Shimpi, N.G. An efficient photocatalytic degradation of organic dyes under visible light using zinc stannate (Zn2SnO4) nanorods prepared by microwave irradiation. Nano Struct. Nano Objects 2020, 21, 100410. [Google Scholar] [CrossRef]
- Rovisco, A.; Branquinho, R.; Martins, J.; Oliveira, M.J.; Nunes, D.; Fortunato, E.; Martins, R.; Barquinha, P. Seed-Layer Free Zinc Tin Oxide Tailored Nanostructures for Nanoelectronic Applications: Effect of Chemical Parameters. ACS Appl. Nano Mater. 2018, 1, 3986–3997. [Google Scholar] [CrossRef]
- Annamalai, A.; Carvalho, D.; Wilson, K.C.; Lee, M.-J. Properties of hydrothermally synthesized Zn2SnO4 nanoparticles using Na2CO3 as a novel mineralizer. Mater. Charact. 2010, 61, 873–881. [Google Scholar] [CrossRef]
- Rovisco, A.; Branquinho, R.; Martins, J.; Fortunato, E.; Martins, R.; Barquinha, P. Growth Mechanism of Seed-Layer Free ZnSnO3 Nanowires: Effect of Physical Parameters. Nanomaterials 2019, 9, 1002. [Google Scholar] [CrossRef] [PubMed]
- Rovisco, A. Solution-Based Zinc-Tin Oxide Nanostructures: From Synthesis to Applications. Ph.D. Thesis, Universidade NOVA de Lisboa, Lisboa, Portugal, 2019. [Google Scholar]
- Barrocas, B.; Sério, S.; Rovisco, A.; Melo Jorge, M.E. Visible-Light Photocatalysis in Ca0.6Ho0.4MnO3 Films Deposited by RF-Magnetron Sputtering Using Nanosized Powder Compacted Target. J. Phys. Chem. C 2014, 118, 590–597. [Google Scholar] [CrossRef]
- Zhao, Q.; Deng, X.; Ding, M.; Huang, J.; Ju, D.; Xu, X. Synthesis of hollow cubic Zn2SnO4 sub-microstructures with enhanced photocatalytic performance. J. Alloys Compd. 2016, 671, 328–333. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rovisco, A.; Branquinho, R.; Martins, R.; Fortunato, E.; Barquinha, P. Microwave-Assisted Hydrothermal Synthesis of Zn2SnO4 Nanostructures for Photocatalytic Dye Degradation. Mater. Proc. 2021, 4, 92. https://doi.org/10.3390/IOCN2020-07850
Rovisco A, Branquinho R, Martins R, Fortunato E, Barquinha P. Microwave-Assisted Hydrothermal Synthesis of Zn2SnO4 Nanostructures for Photocatalytic Dye Degradation. Materials Proceedings. 2021; 4(1):92. https://doi.org/10.3390/IOCN2020-07850
Chicago/Turabian StyleRovisco, Ana, Rita Branquinho, Rodrigo Martins, Elvira Fortunato, and Pedro Barquinha. 2021. "Microwave-Assisted Hydrothermal Synthesis of Zn2SnO4 Nanostructures for Photocatalytic Dye Degradation" Materials Proceedings 4, no. 1: 92. https://doi.org/10.3390/IOCN2020-07850
APA StyleRovisco, A., Branquinho, R., Martins, R., Fortunato, E., & Barquinha, P. (2021). Microwave-Assisted Hydrothermal Synthesis of Zn2SnO4 Nanostructures for Photocatalytic Dye Degradation. Materials Proceedings, 4(1), 92. https://doi.org/10.3390/IOCN2020-07850









