1. Introduction
Recently, with the entry of the new millennium, for the production of lead–acid batteries, the use of secondary refined lead reached 60–66% of the total lead [
1]. Recycling used lead batteries saves natural resources, consumes less energy to produce lead, and significantly avoids air and water pollution. The complex composition of the spent lead paste creates many difficulties in its processing into lead through a process of energy-intensive decomposition and desulfurization [
2,
3]. Several techniques have been developed to save energy by creating industrial facilities in order to create a method for saving energy and controlling the emissions of total solid particles in lead production [
1,
4]. The hydrometallurgical methods of battery recycling are more widely used [
5,
6]. They involve the oxidation of lead waste by treatment with Na₂CO₃, (NH
4)
2CO
3 or NaOH. The received lead carbonate, hydroxide or hydroxycarbonate is subjected to pyrometallurgical treatment at lower temperatures (400–650 °C) compared to pyrometallurgical methods (900–1400 °C) [
7,
8,
9,
10,
11]. In recent years, research has been based on the need to develop an efficient, low-cost environmental technology for recycling waste lead paste. Experiments to extract Pb from used lead paste by treatment with aqueous citric acid solution lead to the generation of a lead citrate precursor, which can easily be converted to PbO for direct production of lead pastes for batteries. Controlling the calcination process may lead to variations in the microstructure and the formation of micro- to nanosized powders—a new perspective in the development of lead–acid battery technology [
12,
13].
The purpose of our research is to find an optimal method for the recycling of used lead–acid batteries in order to recover lead from positive (PAM) and negative (NAM) active masses and obtain lead oxide powders for direct application in the formation of lead–acid pastes.
2. Materials and Methods
Desulfurization and leaching were performed in one step by simultaneously adding aqueous solutions of C6H5Na3O7.2H2O (0.5 mol/L) and C6H8O7 (0.8 mol/L, 1 mol/L) at varying temperatures (25–100 °C) and heat treatment times (1–2 h). The ratio PbSO4/C6H5Na3O7.2H2O/C6H8O7 was 1:2.5:2.5. Hydrogen peroxide was added to the samples of the positive active masses as a reducing agent for the conversion of Pb (IV) to Pb (II) at the sample/acid solution/peroxide ratio of 1:1.5:2. The calcination was carried out by heating at 300 °C for 1 h. Initially, the experiments were conducted with chemical purity substances (PbO2, Pb(NO3)2 and PbSO4) and lead oxide powder (85.7% PbO/14.3% Pb) for determining the appropriate conditions to prevent the lead losses during its recovery. The spent positive and negative active masses had the following chemical compositions: NAM: 68.9% PbSO4, 24.56% Pb, 2.98% PbO; PAM: 33.08% PbSO4, 66.37% PbO2.
The phase composition was determined by XRD, using a Philips PW 1030 X-ray diffractometer with a Bragg–Brentano θ–2θ geometry, CuKα radiation (30 kV, 20 mA) with wavelength λ = 1.5406 Å and scintillation detector. A scanning electron microscope (JEOL JEM-200CX) was used for morphology observation of the samples. The images were taken in secondary electrons mode (SE) at accelerating voltage of 80 keV. DTA/TG analysis was performed by Labsys Evo 1600 Setaram.
3. Results and Discussion
During the experiment, it was found that the final result of desulfurization and leaching of spent active masses of lead batteries is the formation of different phases. These results provoked us to conduct additional research on the preparation of lead salts as a product of recycling. For this purpose, we used chemically pure lead citrate (Pb
3(C
6H
5O
7)
2.3H
2O—99%, Alfa Aesar). Its X-ray served as a standard (
Figure 1a(I), 1b(I)).
Figure 1a shows the diffractograms of lead citrate obtained from chemically pure substances (PbO
2, Pb(NO
3)
2, PbSO
4) under the following conditions: 2 h magnetic stirring, 1 h heating at 40 °C, 0.8 mol/L citric acid solution, and 0.5 mol/L sodium citrate solution + H
2O
2 for Pb (IV). The results confirm the conclusions made so far in the literature on the type of lead citrate [
12,
14]. Therefore, the appearance of a high-intensity peak at ~7.8° 2θ may be associated with the production of Pb(C
6H
6O
7).H
2O. As can be seen, in the case of starting from lead oxide powder, PbO
2 and Pb(NO
3)
2, lead citrate monohydrate was formed (
Figure 1a(II, III, IV)). Their diffractograms did not correspond to the standard. When lead sulfate was used as raw material after desulfurization and leaching, it did not dissolve completely, and its peaks are registered in the diffractogram, as well as those of two types of lead citrate (
Figure 1a(V)).
Figure 1b presents the results of XRD analysis of selected samples of positive (PAM) and negative (NAM) active mass after recycling, as well as lead citrate obtained by following the methodology of patent SU 1159958, which reports the formation of lead citrate from lead nitrate and citric acid [
15]. The results are somewhat consistent with those obtained using chemically pure products. In the case of desulfurization and leaching of PAM, in contrast to pure PbO
2, Pb
3(C
6H
5O
7)
2.3H
2O was formed while the X-ray pattern of NAM under one of the same sets of conditions (temperature and heat treatment time) shows the formation of lead monohydrate (
Figure 1b(II, III)). A mixture of two citrates was observed in the diffraction pattern of the final synthesis product carried out according to the above patent (
Figure 1b(IV)).
A negative active mass was treated to compare the results obtained using citric acid solutions with different concentrations. Lead citrate trihydrate was obtained when using a solution with a lower concentration (0.8 mol/L), temperature (40 °C) and heat treatment time (1 h). After calcination, the XRD diagram shows that the main phase formed is β-PbO and a small amount of Pb (
Figure 2a(I,II)), and the absence of a peak at 29.69° 2θ proves that the desulfurization was complete. At a higher concentration (1 mol/L) of the acid solution, the desulfurization was not complete, and residual lead sulfate was present in addition to lead citrate monohydrate. In the received lead oxide powder, the base phase is again β-PbO and a small amount of Pb. In this case, α-PbO was formed, along with oxide sulfates (PbO.PbSO
4 and 4PbO.PbSO
4) in significant amounts (
Figure 2a(III,IV)).
All reported results were recorded starting from pure chemicals, positive active mass, or negative active mass. They gave us the basic guidelines on how to conduct the experiment, approaching the real conditions of recycling lead–acid batteries. For this purpose, we mixed 1:1 RAM and NAM (sample NP-3) and applied a methodology established on the basis of the optimal conditions achieved so far.
Figure 2b shows the XRD diagrams of the mixed sample before and after desulfurization and leaching (
Figure 2b(I,II)). The obtained lead citrate is Pb
3(C
6H
5O
7)
2.3H
2O without the presence of PbO
2 and PbSO
4. After calcination at 300° C for 1 h, high-purity lead oxide powder was obtained for direct application in the formation of active masses for lead–acid batteries (
Figure 2b(III)).
The crystallite sizes (t) were determined by Scherer’s formula: t = κλ/Bcosθ, where k is shape factor (k = 0.9 for spherical crystals with cubic symmetry), λ (Å) is the wavelength, θ is the diffraction angle of the peak and B (rad) is the line broadening at the full width at half maximum (FWHM) values of the peaks. The measured crystallite sizes of the two main phases of obtained lead oxide powders after calcination of samples NAM-1 and NP-3 were β-PbO(111)—46 nm, Pb(111)—57 nm and β-PbO(111)—37 nm, Pb(111)—49 nm, respectively.
These results and the conclusions reached regarding the type of citrate can to some extent be confirmed by the DTA/TG analysis.
Figure 3a presents the results of the DTA analysis of Pb
3(C
6H
5O
7)
2.3H
2O used as a reference in our studies. The endoeffect at 135.4° C, as well as 4.27% weight loss, may be related to the dehydration of lead crystal hydrate. As can be seen for the citrate obtained by leaching PAM at approximately the same temperature (138.8 °C), an endoeffect was observed again, as well as one with less heat absorption at 165.6 °C, which at this stage is still difficult to explain (
Figure 3b). In this case, in the XRD analysis, in addition to lead citrate, PbO
2 and PbSO
4 were registered in small quantities and additional transformations were obviously taking place, which is evidenced not only by the manifestation of the additional endoeffect but also by greater weight loss (6.44%). The linear character of the curve of the lead citrate obtained from chemically pure Pb(NO
3)
2 shows that the anhydrous form of lead citrate is formed during its synthesis from chemically pure products, which on the other hand differs from the initial conclusion based on the data from the literature (
Figure 3c).
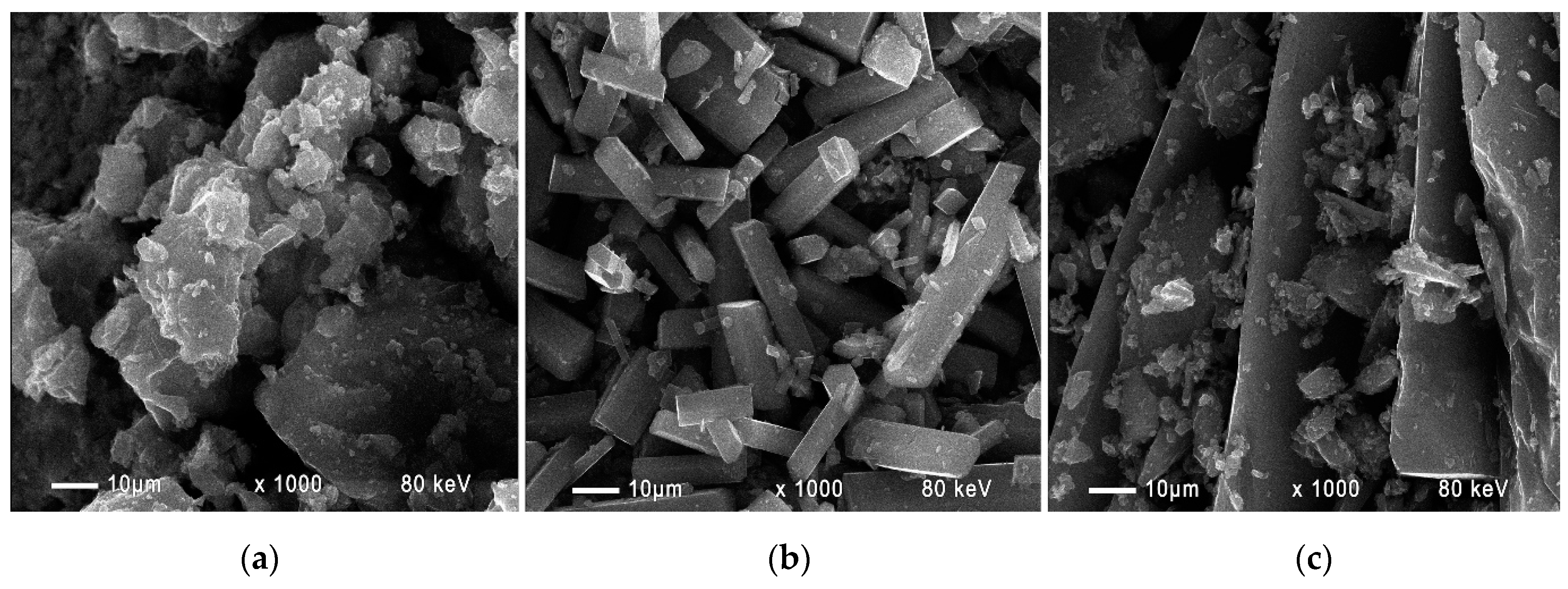
The conducted SEM analysis allowed us to determine the morphological features of the obtained lead citrate. The images show that the crystallization is complete. The synthesized Pb
3(C
6H
5O
7)
2.3H
2O crystals fused together by desulfurization and leaching of PAM to form druses (
Figure 4a). In the synthesis of lead citrate from a mixture of NAM and PAM (NP-3), long prismatic crystals with a length of up to 50 μm and a cross-section of ~5 μm were formed (
Figure 4b). In the lead citrate obtained under patent SU 1159958, where we established by XRD the presence of both types of citrate, the crystals with different shapes fused together to form druses (
Figure 4c).
These results provide guidelines for further studies of lead citrate types, underscoring the importance of their crystallographic and morphological characteristics for the formation of lead oxide powders with optimal chemical composition and crystallite sizes for use in the production of lead–acid batteries with high storage capacity and long lifespan.