Development of Metal Nanoparticles Based Sensing Platform for Lead in Aqueous Samples †
Abstract
:1. Introduction
2. Materials
3. Method
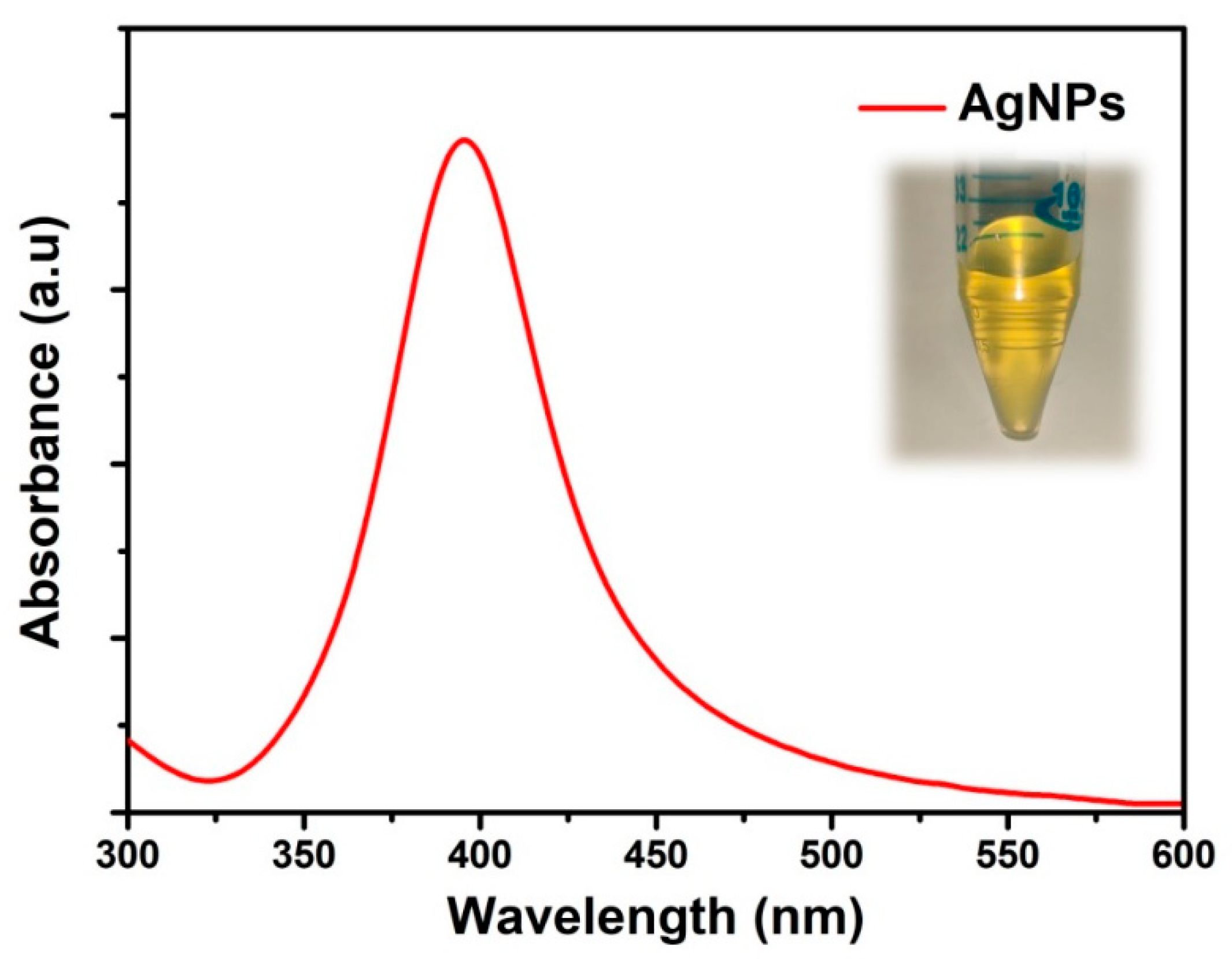
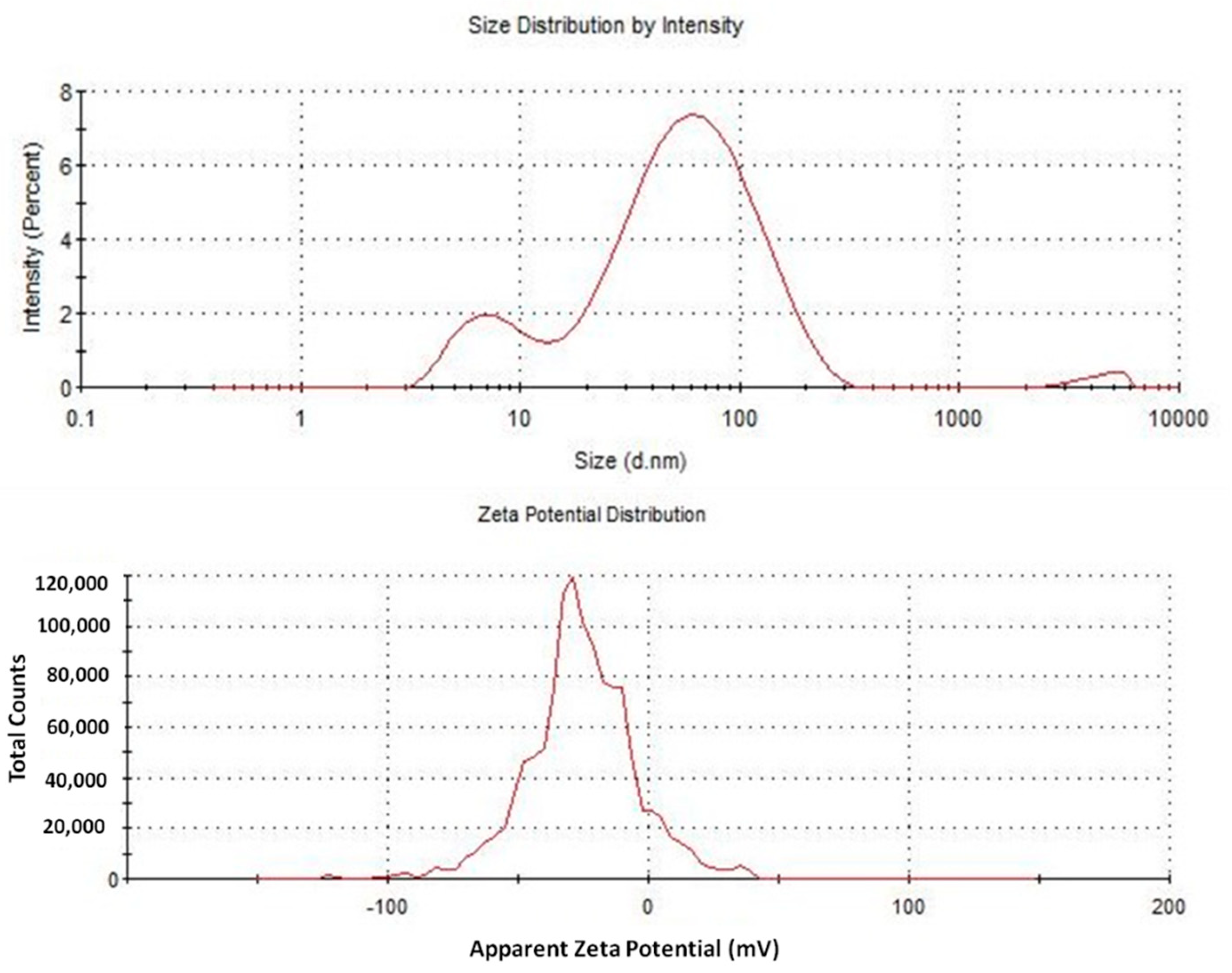
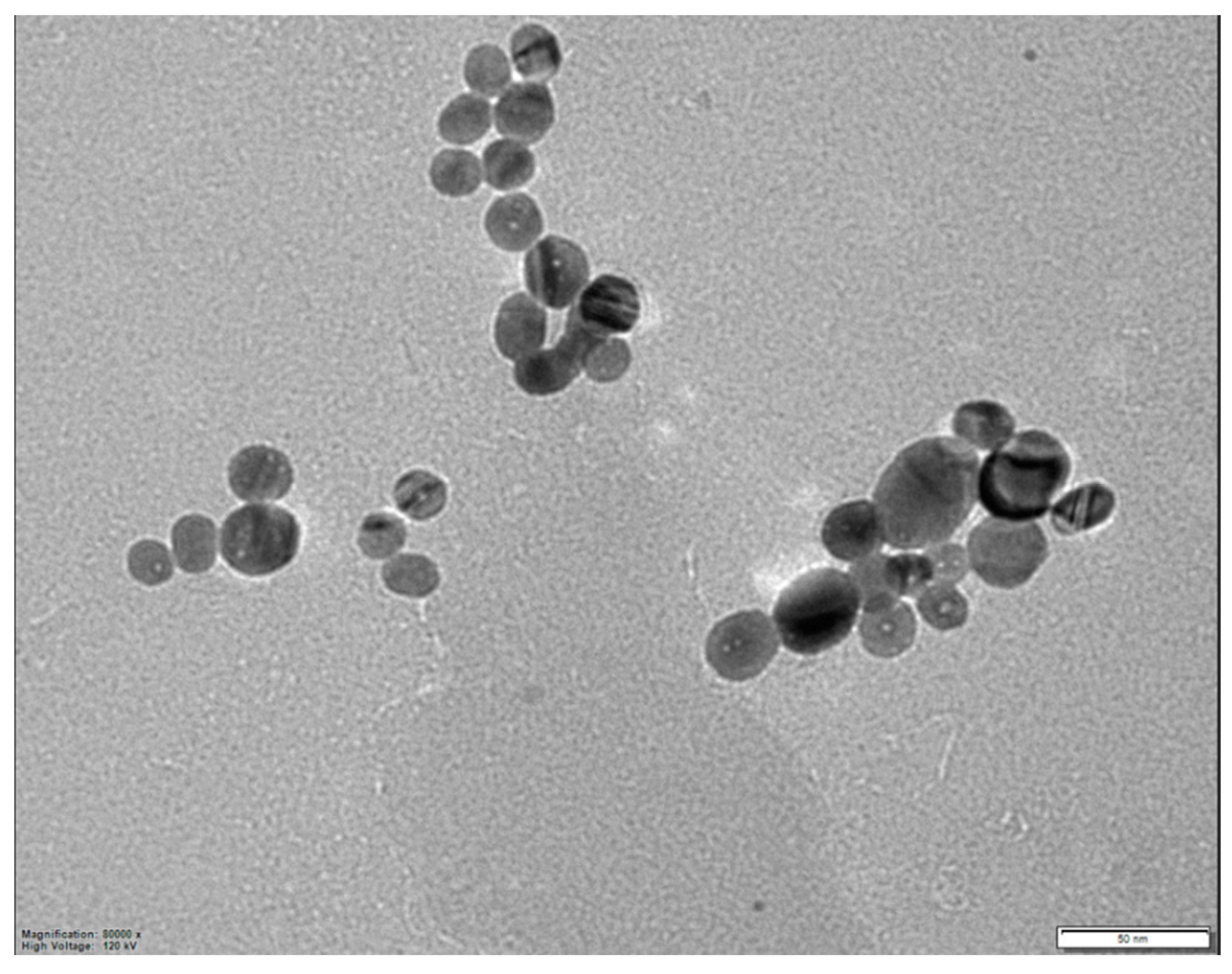
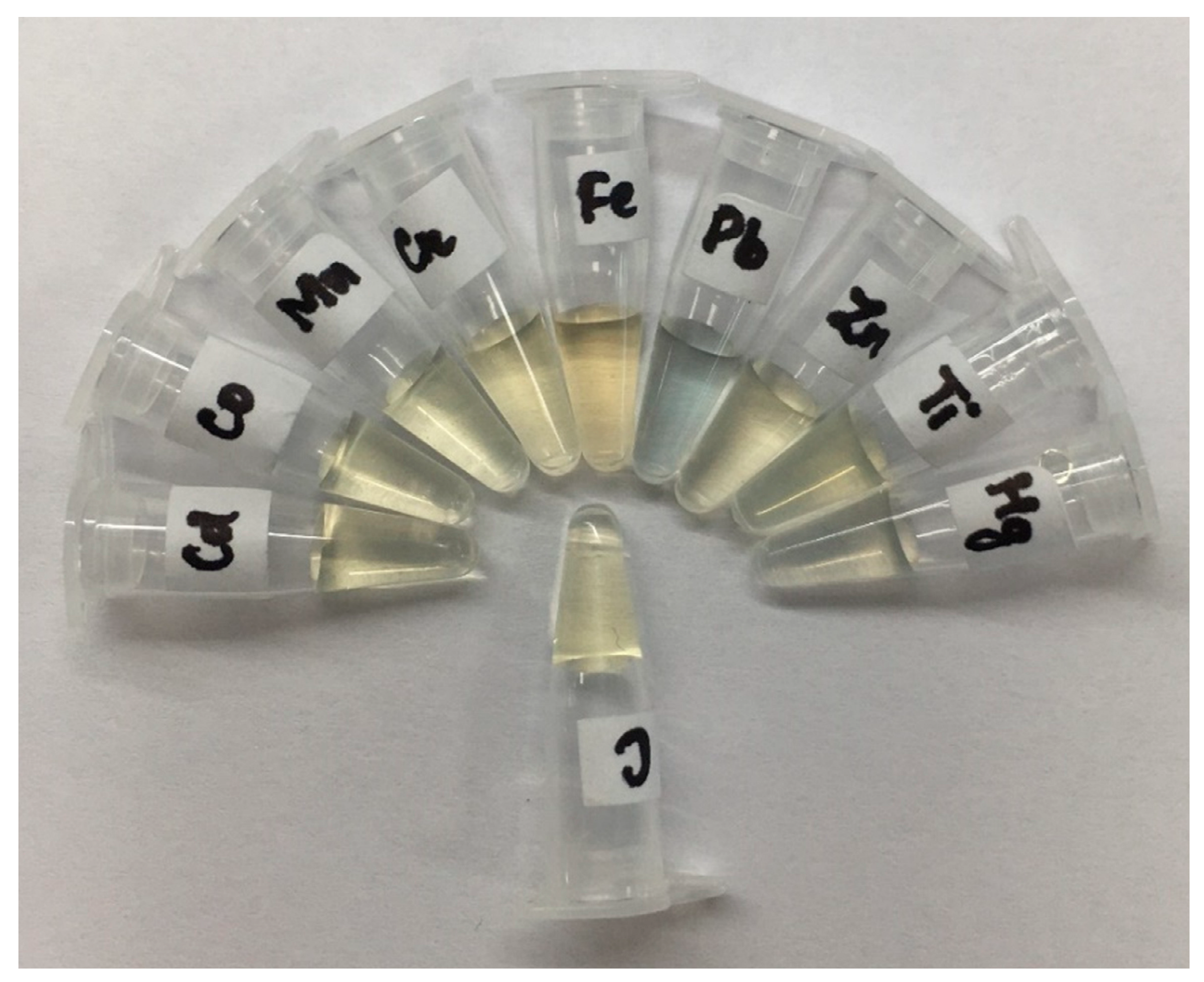
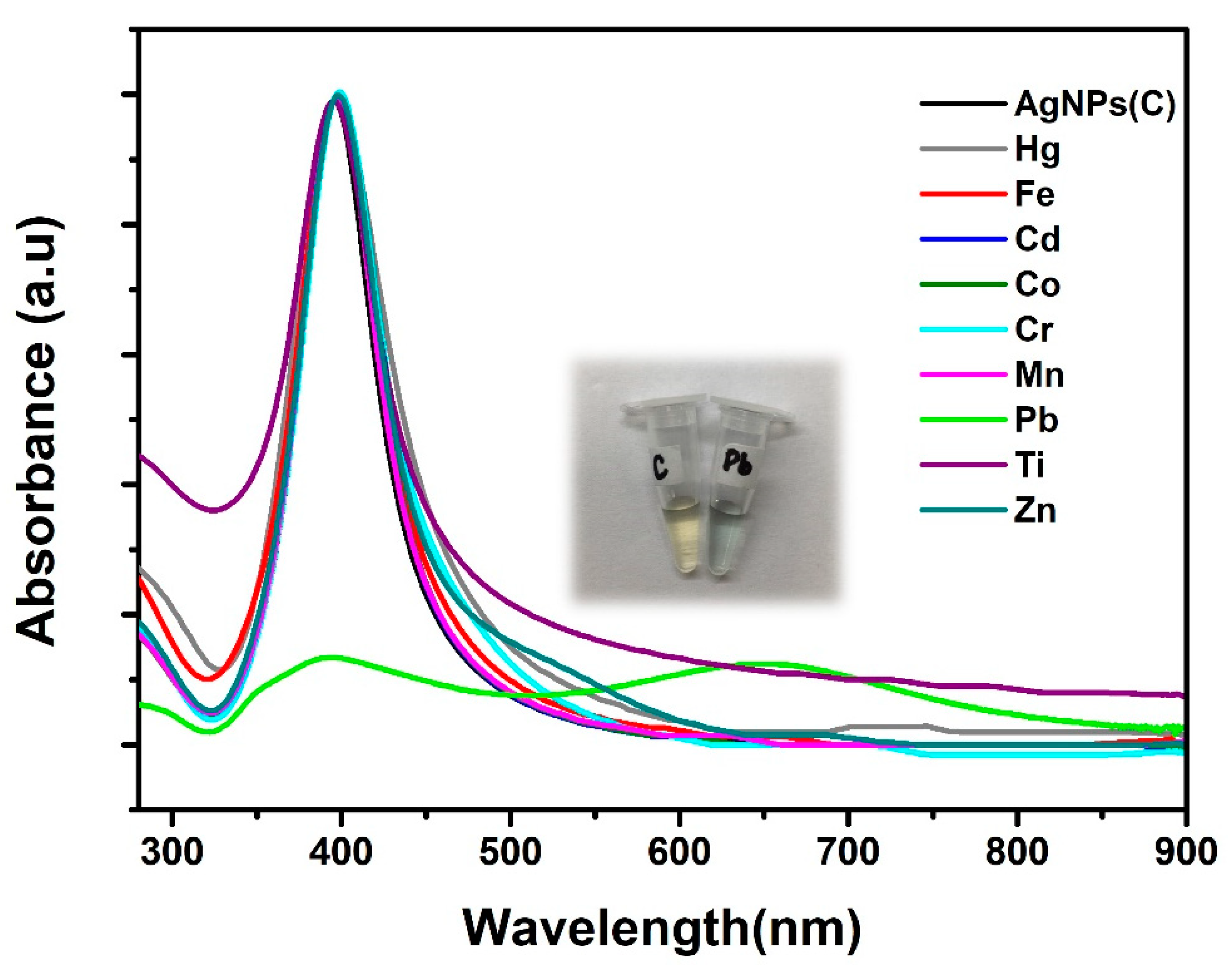
4. Results and Discussion
5. Conclusions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
References
- Virk, R.K.; Shafique, M.; Vaid, K.; Verma, S.; Bansal, M.P.; Garg, M.L.; Mohanty, B.P. Tracing biodistribution of essential and toxic elements in rat liver through PIXE. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 477, 91–97. [Google Scholar] [CrossRef]
- Dai, X.; Wu, S.; Li, S. Progress on electrochemical sensors for the determination of heavy metal ions from contaminated water. J. Chin. Adv. Mater. Soc. 2018, 6, 91–111. [Google Scholar] [CrossRef]
- Kumar, A.; MMS, C.-P.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; S Kumar, S.; A Khan, S. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef] [PubMed]
- Organization, W.H. International Lead Poisoning Prevention Week 2019 Campaign Resource Package; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Vaid, K.; Dhiman, J.; Sarawagi, N.; Kumar, V. Experimental and computational study on the selective interaction of functionalized gold nanoparticles with metal ions: Sensing prospects. Langmuir 2020, 36, 12319–12326. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zeinu, K.M.; Gao, S.; Liu, B.; Yang, J.; Hu, J. Recent advances and perspective on design and synthesis of electrode materials for electrochemical sensing of heavy metals. Energy Environ. Mater. 2018, 1, 113–131. [Google Scholar] [CrossRef]
- Sang, S.; Li, D.; Zhang, H.; Sun, Y.; Jian, A.; Zhang, Q.; Zhang, W. Facile synthesis of AgNPs on reduced graphene oxide for highly sensitive simultaneous detection of heavy metal ions. RSC Adv. 2017, 7, 21618–21624. [Google Scholar] [CrossRef]
- Aadil, K.R.; Pandey, N.; Mussatto, S.I.; Jha, H. Green synthesis of silver nanoparticles using acacia lignin, their cytotoxicity, catalytic, metal ion sensing capability and antibacterial activity. J. Environ. Chem. Eng. 2019, 7, 103296. [Google Scholar] [CrossRef]
- Preciado-Flores, S.; Wheeler, D.A.; Tran, T.M.; Tanaka, Z.; Jiang, C.; Barboza-Flores, M.; Qian, F.; Li, Y.; Chen, B.; Zhang, J.Z. SERS spectroscopy and SERS imaging of Shewanella oneidensis using silver nanoparticles and nanowires. Chem. Commun. 2011, 47, 4129–4131. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaid, K.; Dhiman, J.; Sarawagi, N.; Kumar, S.; Kumar, V. Development of Metal Nanoparticles Based Sensing Platform for Lead in Aqueous Samples. Mater. Proc. 2021, 4, 61. https://doi.org/10.3390/IOCN2020-07852
Vaid K, Dhiman J, Sarawagi N, Kumar S, Kumar V. Development of Metal Nanoparticles Based Sensing Platform for Lead in Aqueous Samples. Materials Proceedings. 2021; 4(1):61. https://doi.org/10.3390/IOCN2020-07852
Chicago/Turabian StyleVaid, Kalyan, Jasmeen Dhiman, Nikita Sarawagi, Suresh Kumar, and Vanish Kumar. 2021. "Development of Metal Nanoparticles Based Sensing Platform for Lead in Aqueous Samples" Materials Proceedings 4, no. 1: 61. https://doi.org/10.3390/IOCN2020-07852
APA StyleVaid, K., Dhiman, J., Sarawagi, N., Kumar, S., & Kumar, V. (2021). Development of Metal Nanoparticles Based Sensing Platform for Lead in Aqueous Samples. Materials Proceedings, 4(1), 61. https://doi.org/10.3390/IOCN2020-07852






