Abstract
Effects of co-addition of CuBr2 and NaCl to CH3NH3PbI3(Cl) perovskite solar cells were investigated on the photovoltaic properties and microstructures. Short-circuit current densities and conversion efficiencies were improved by the simultaneous addition of CuBr2 and NaCl. In addition, the efficiencies of the devices were maintained after 10 weeks. The perovskite structure changed from a tetragonal to a cubic system by the addition of Na, which resulted in the improvement of the perovskite crystal’s stabilization.
1. Introduction
Perovskite solar cells are widely studied, and the partial substitution method with different elements and molecules is often used to improve the properties of perovskite solar cells [1,2,3,4]. Organic−inorganic metal halide perovskites have a cubic structure with a general formula ABX3, where A is an organic cation, B is a divalent metal ion, and X is a halide ion [5]. Doped perovskite structures have been obtained by adding various compounds to the perovskite precursor solutions, and the various dopants investigated are as follows: CH(NH2)2+ [6,7,8,9], C(NH2)3+ [10,11], CH3CH2NH3 [12], Cs+ [7,13], Rb+ [7,14], and K+ [15,16,17] at CH3NH3+ (MA+) sites, Sn2+ [18,19] or Sb3+ [20,21] at Pb2+ sites, and Br− and Cl− at I− sites [22,23,24].
In previous studies, perovskite crystals have been synthesized from MAPbI3−xClx mixed precursor solutions with MAI and PbCl2 in the ratio of 3:1 [25,26,27], and the highest conversion efficiency was obtained for the cells fabricated at 140 °C [26]. Although annealing at high temperatures above 200 °C was possible by using polysilanes in chlorobenzene [17,28], perovskite grains may decompose even at 150 °C, and MAPbI3 changes into PbI2 by MA desorption. Another approach to improve the MAPbI3−xClx cells is by adding a small amount of excess PbI2 to the MAPbI3−xClx precursor solutions [29]. The recombination of electrons and holes was suppressed by the formation of a small amount of PbI2, and the external quantum efficiencies increased. Stabilities of the PbI2-added cells were also improved [30], and Cl was an important element to improve the stability, which was shown by the constant Cl content in the MAPbI3−xCl cells after several weeks [29,30].
The short-circuit current density was also increased by the simultaneous addition of alkali metal iodides (NaI, KI, RbI, or CsI) and CuBr2 to mixed perovskite precursor solutions with MAI, PbCl2, and PbI2 [31]. It was suggested that the lattice constant decreased by adding CuBr2 because of the smaller ionic radius of Cu2+ compared with that of Pb2+. An increase of the density of states owing to the alkali metal elements was confirmed by first-principles calculations, which contributed to the increase of charge generation by light absorption in the shorter wavelength range as compared with the band gap energy of the perovskite. In the co-addition study, it was necessary to optimize adding an amount of CuBr2 and alkali metal iodides to further improve the photovoltaic characteristics of the perovskite devices. However, increasing the amount of Cu leads to lattice defects that break the bond between Cu/Pb and halogen due to the smaller ionic radius of Cu2+ than that of Pb2+. The addition of alkali metal elements is effective in stabilizing the perovskite solar cells because of the more stable alkali metal elements compared with MA. Since the Cu−Cl bond is shorter than the Cu−Br bond for MA2CuCl2Br2 with two-dimensionality [32], it is considered that the smaller ionic radii such as that of Na/Cl with excellent solubility is effective for the perovskite formation.
The purpose of the present study is to fabricate NaCl/CuBr2 co-added MAPbI3−xClx photovoltaic devices and to characterize the photovoltaic properties and crystal structures. The additive quantities of NaCl and CuBr2 to the perovskite precursor solutions are optimized to improve the photovoltaic performance and stability of the perovskite solar cells [33]. Then, the effects of Na addition to Cu-modified perovskite are investigated by analyzing the changes in the conversion efficiencies and the microstructures. The tolerance factor approaches 1 by introducing Cu2+ at the position of Pb2+ because of the smaller ionic radius of Cu2+ (0.73 Å) compared with that of Pb2+ (1.19 Å). Thus, a more stable perovskite structure is expected to be formed by adding CuBr2. Gibbs-free energies were also calculated and compared.
2. Materials and Methods
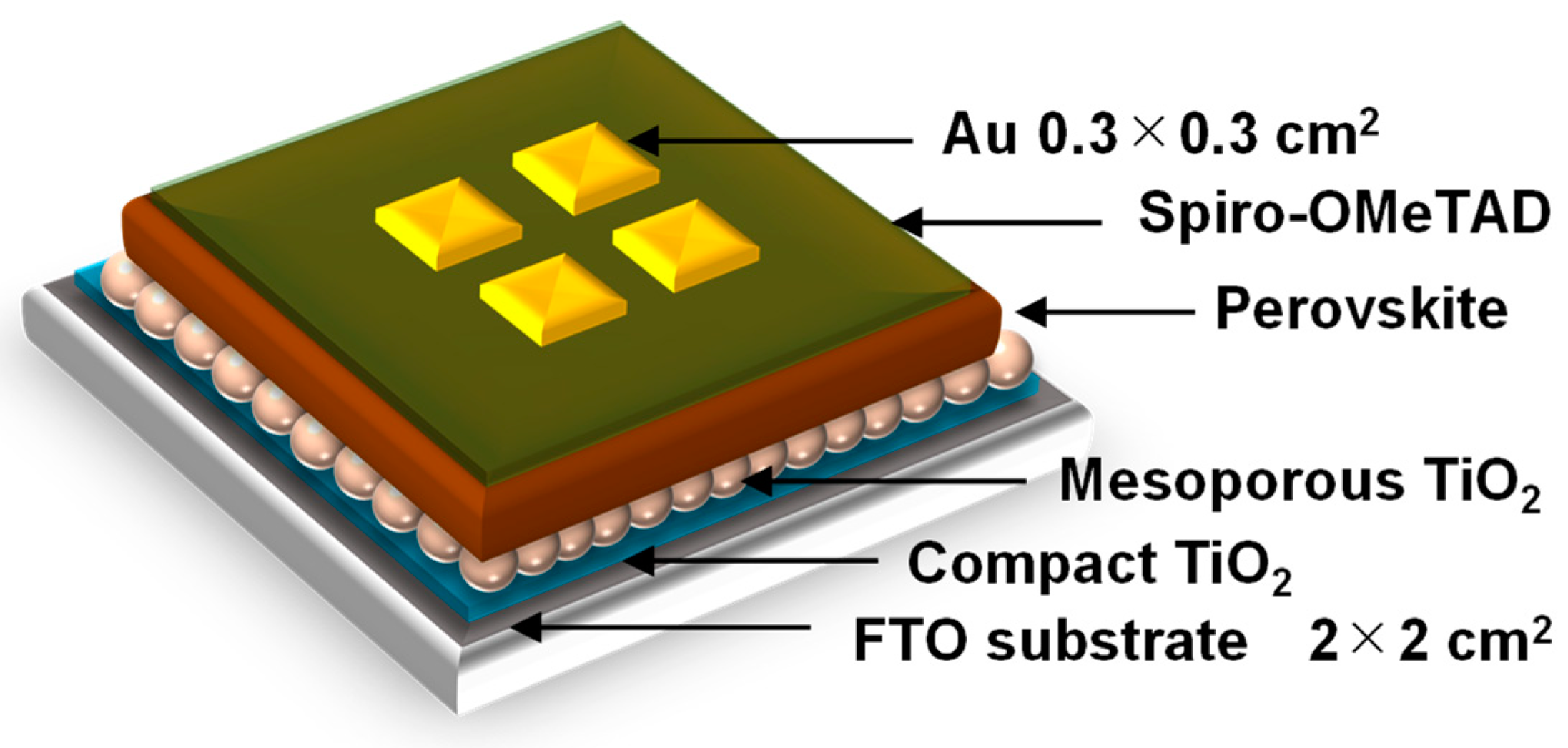
A schematic illustration of the present perovskite photovoltaic cells is shown in Figure 1. F-doped tin oxide (FTO) substrates were cleaned in an ultrasonic bath with acetone and methanol and dried under nitrogen gas. The TiO2 (0.15 and 0.30 M) precursor solutions were prepared from titanium diisopropoxide bis(acetyl acetonate) (Sigma-Aldrich, Tokyo, Japan, 0.055 and 0.11 mL) with 1-butanol (1 mL). The 0.15 M TiO2 precursor solution was spin-coated on the FTO substrate at 3000 rpm for 30 s, and the coated substrate was then annealed at 125 °C for 5 min. The 0.30 M TiO2 precursor solution was spin-coated on the TiO2 layer at 3000 rpm for 30 s, and the resulting substrate was annealed at 125 °C for 5 min. The process to form the 0.30 M precursor layer was performed twice. Then, the FTO substrate was sintered at 550 °C for 30 min to form a compact TiO2 layer [33,34,35]. To form the mesoporous TiO2 layer, a TiO2 paste was prepared from the TiO2 powder (Aerosil, Tokyo, Japan, P-25) with poly(ethylene glycol) (Nacalai Tesque, Kyoto, Japan, PEG #20000) in ultrapure water. The solution was mixed with acetylacetone (Wako Pure Chemical Industries, Osaka, Japan, 10 mL) and surfactant (Sigma-Aldrich, Triton X-100, 5 mL) for 30 min and then allowed to stand for 24 h to remove bubbles from the solution. Then, the TiO2 paste was spin-coated on the compact TiO2 layer at 5000 rpm for 30 s. The resulting cell was heated at 125 °C for 5 min and then annealed at 550 °C for 30 min to form the mesoporous TiO2 layer.
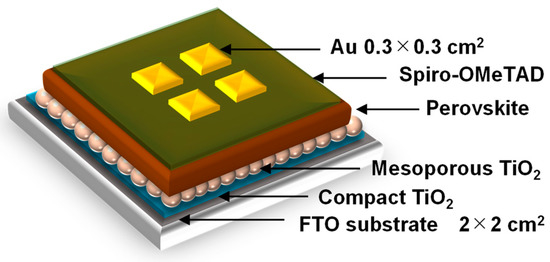
Figure 1.
Schematic illustration of the present perovskite photovoltaic devices.
To prepare the perovskite compounds, mixed solutions of CH3NH3I (2.4 M, Showa Chemical, Tokyo, Japan), PbCl2 (0.8 M, Sigma-Aldrich, St. Louis, MO, USA), and PbI2 (0.08 M, Sigma-Aldrich) in DMF (Sigma-Aldrich, 500 mL) were prepared for the standard cell [33]. Pb or MA in the perovskite structure was expected to be substituted by Cu or alkali metal elements, respectively. These perovskite solutions were then introduced into the TiO2 mesopores by spin-coating at 2000 rpm for 60 s, which is followed by annealing at 140 °C for 10 min under 27 °C and 40% humidity in air.
A hole-transport layer was prepared by spin-coating. A solution of spiro-OMeTAD (Wako Pure Chemical Industries, Osaka, Japan, 50 mg) in chlorobenzene (Wako Pure Chemical Industries, 0.5 mL) was mixed with a solution of lithium bis(tri-fluoromethylsulfonyl)imide (Li-TFSI, Tokyo Chemical Industry, Tokyo, Japan, 260 mg) in acetonitrile (Nacalai Tesque, Kyoto, Japan, 0.5 mL) for 24 h. The former solution with 4-tertbutylpyridine (Sigma-Aldrich, 14.4 mL) was mixed with the Li-TFSI solution (8.8 mL) for 30 min at 70 °C. Then, the spiro-OMeTAD solution was spin-coated on the perovskite layer at 4000 rpm for 30 s. All procedures were performed in ambient air. Finally, gold (Au) electrodes were evaporated as top electrodes using a metal mask for the patterning. Layered structures of the prepared photovoltaic cells are denoted as FTO/TiO2/perovskite/spiro-OMeTAD/Au. The prepared perovskite photovoltaic devices were stored at 22 °C and ~30% humidity.
The photovoltaic properties of the fabricated solar cells were investigated by current density−voltage (J−V) characteristics and external quantum efficiency (EQE). Microstructures of the thin films were investigated by X-ray diffraction (XRD), optical microscopy, and scanning electron microscopy (SEM)/energy dispersive spectroscopy (EDS).
3. Results and Discussion
Table 1 shows photovoltaic parameters of the present perovskite photovoltaic devices with added NaCl and CuBr2 under air mass 1.5 sunlight illumination [33]. The device with only 2% CuBr2 added without NaCl provided a short-circuit current density (JSC) of 18.9 mA cm−2, an open-circuit voltage (VOC) of 0.952 V, a fill factor (FF) of 0.655, and a conversion efficiency (η) of 11.8%. The JSC was increased from 18.9 to 22.7 mA cm−2 by the simultaneous addition of 2% CuBr2 and 2% NaCl, and the η increased to 14.5%. Hysteresis of the current density-voltage curves were decreased by the co-addition of 2% CuBr2 and 2% NaCl, which contributed to suppressing the recombination between a hole and an electron. For the 5% CuBr2-added device, the carrier transport property and the quality of the perovskite layer were improved by increasing the amount of NaCl added, which resulted in an improvement of η.

Table 1.
Measured photovoltaic parameters of the perovskite photovoltaic devices.
Stability measurements of the photovoltaic parameters up to 10 weeks were carried out. The η of the (2% NaCl + 2% CuBr2)-added device remained almost constant for 10 weeks. Although most of Cl were evaporated during the annealing process, a small amount of Cl remained in the perovskite layers. The remaining Cl in the perovskite layer played a critical role in the perovskite grain growth, which resulted in a few defects in perovskite thin films, and also led to stability. The higher VOC and FF of the (7% CuBr2 + 5% NaCl)-added device were maintained for 10 weeks in comparison with those of the (5% CuBr2 + 5% NaCl)-added device, which caused a structural transition from a tetragonal to a cubic system by adding excess CuBr2, and suppressed recombination between a hole and an electron, owing to the pinhole decrease.
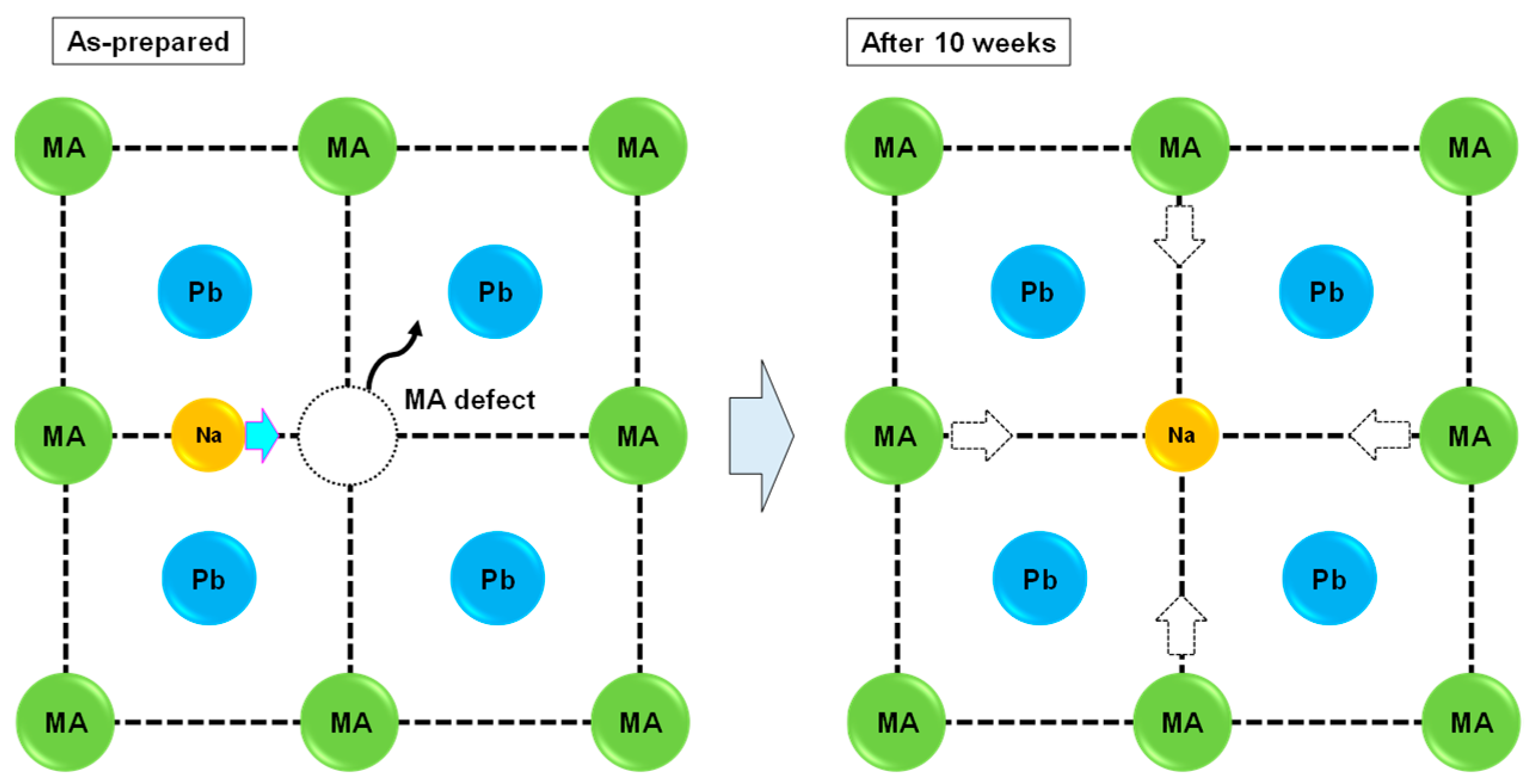
The partial Na would transfer into the MA vacancy site after 10 weeks, which caused the structural transformation from a pseudo-cubic system to a cubic system, as shown in Figure 2. This structural change indicated that a small amount of Na improved the stability of the photovoltaic properties. Although the present perovskite structures exhibited a nearly cubic structure from the lattice constant, weak diffraction peaks corresponding to tetragonal symmetry were observed at ~23°. This structure was classified as pseudo-cubic [5,36,37] of the a/c axis, which was 1 in the present work. This pseudo-cubic structure was transformed into perfect cubic symmetry by adding excess CuBr2, which was confirmed by the disappearance of the diffraction peaks at ~23° for the 10% CuBr2-added devices.
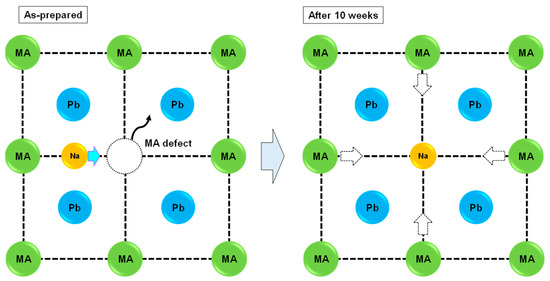
Figure 2.
Stability mechanism for the Na-added perovskite crystals.
4. Conclusions
Effects of co-addition of NaCl and CuBr2 to MAPbI3 were investigated on the microstructures and photovoltaic properties. A smooth perovskite layer was obtained under the ambient air, and the JSC was improved by the simultaneous addition of CuBr2 and NaCl. The conversion efficiency of 14% was maintained for the (2% CuBr2 + 2% NaCl)-added device even after 10 weeks. The perovskite structure changed from a pseudo-cubic (tetragonal) to a cubic system by adding Na. The Na atoms exist at the interstitial sites and are transferred to the MA vacancy sites after several weeks, which resulted in the improvement in the perovskite crystal’s stabilization. The photovoltaic performance of the device co-added with CuBr2 and NaCl was superior to that of the device added with CuBr2 and NaI.
Author Contributions
Conceptualization, N.U.; methodology, N.U. and T.O.; formal analysis, N.U. and T.O.; investigation, N.U.; resources, N.U. and T.O.; data curation, N.U. and T.O.; writing—original draft preparation, N.U. and T.O.; project administration, N.U. and T.O.; funding acquisition, N.U. and T.O. All authors have read and agreed to the published version of the manuscript.
Funding
A part of the present study was supported by JSPS KAKENHI Grant Number 19J15017 and the Super Cluster Program of the Japan Science and Technology Agency (JST).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Pearson, A.J.; Divitini, G.; Cacovich, S.; Philippe, B.; Rensmo, H.; Ducati, C.; Friend, R.H.; Stranks, S.D. Potassium-and rubidium-passivated alloyed perovskite films: Optoelectronic properties and moisture stability. ACS Energy Lett. 2018, 3, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yao, Z.; Yu, F.; Yang, D.; Liu, S.F. Alkali metal doping for improved CH3NH3PbI3 perovskite solar cells. Adv. Sci. 2018, 5, 1700131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; You, S.; Huang, J.; Yuan, L.; Xiao, Z.Y.; Cao, Y.; Cheng, N.; Hu, L.; Liu, J.F.; Yu, B.H. Molecular modulator for stable inverted planar perovskite solar cells with efficiency enhanced by interface engineering. J. Mater. Chem. C 2019, 7, 9735–9742. [Google Scholar] [CrossRef]
- Lu, Y.A.; Chang, T.H.; Wu, S.H.; Liu, C.C.; Lai, K.W.; Chang, Y.C.; Chang, Y.C.; Lu, H.C.; Chu, C.W.; Ho, K.C. Coral-like perovskite nanostructures for enhanced light-harvesting and accelerated charge extraction in perovskite solar cells. Nano Energy 2019, 58, 138–146. [Google Scholar] [CrossRef]
- Oku, T. Crystal structures of perovskite halide compounds used for solar cells. Rev. Adv. Mater. Sci. 2020, 59, 264–305. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, H.; Yuan, H.; Yang, Z.; Fan, J.Z.; Kim, J.; Voznyy, O.; Gong, X.; Quan, L.N.; Tan, C.S.; et al. Perovskite seeding growth of formamidinium-lead-iodide-based perovskites for efficient and stable solar cells. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Philippe, B.; Saliba, M.; Baena, J.-P.C.; Cappel, U.B.; Cruz, S.H.T.; Grätzel, M.; Hagfeldt, A.; Rensmo, H. Chemical distribution of multiple cation (Rb+, Cs+, MA+, and FA+) perovskite materials by photoelectron spectroscopy. Chem. Mater. 2017, 29, 3589–3596. [Google Scholar] [CrossRef]
- Ono, L.K.; Perez, E.J.J.; Qi, Y. Progress on perovskite materials and solar cells with mixed cations and halide anions. ACS Appl. Mater. Interfaces 2017, 9, 30197–30246. [Google Scholar] [CrossRef]
- Suzuki, A.; Kato, M.; Ueoka, N.; Oku, T. Additive effect of formamidinium chloride in methylammonium lead halide compound-based perovskite solar cells. J. Electron. Mater. 2019, 48, 3900–3907. [Google Scholar] [CrossRef]
- Jodlowski, A.D.; Roldán-Carmona, C.; Grancini, G.; Salado, M.; Ralaiarisoa, M.; Ahmad, S.; Koch, N.; Camacho, L.; Miguel, G.; Nazeeruddin, M. Large guanidinium cation mixed with methylammonium in lead iodide perovskites for 19% efficient solar cells. Nat. Energy 2017, 2, 972–979. [Google Scholar] [CrossRef]
- Kishimoto, T.; Suzuki, A.; Ueoka, N.; Oku, T. Effects of guanidinium addition to CH3NH3PbI3-xClx perovskite photovoltaic devices. J. Ceram. Soc. Jpn. 2019, 127, 491–497. [Google Scholar] [CrossRef]
- Nishi, K.; Oku, T.; Kishimoto, T.; Ueoka, N.; Suzuki, A. Photovoltaic characteristics of CH3NH3PbI3 perovskite solar cells added with ethylammonium bromide and formamidinium iodide. Coatings 2020, 10, 410. [Google Scholar] [CrossRef]
- Singh, R.; Sandh, S.; Yadav, H.; Lee, J.J. Stable triple-cation (Cs+−MA+−FA+) perovskite power formation under ambient conditions for hysteresis-free high-efficiency solar cells. ACS Appl. Mater. Interfaces 2019, 11, 29941–29949. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Baena, J.P.C.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Machiba, H.; Oku, T.; Kishimoto, T.; Ueoka, N.; Suzuki, A. Fabrication and evaluation of K-doped MA0.8FA0.1K0.1PbI3(Cl) perovskite solar cells. Chem. Phys. Lett. 2019, 730, 117–123. [Google Scholar] [CrossRef]
- Kandori, S.; Oku, T.; Nishi, K.; Kishimoto, T.; Ueoka, N.; Suzuki, A. Fabrication and characterization of potassium- and formamidinium-added perovskite solar cells. J. Ceram. Soc. Jpn. 2020, 128, 805–811. [Google Scholar] [CrossRef]
- Oku, T.; Kandori, S.; Taguchi, M.; Suzuki, A.; Okita, M.; Minami, S.; Fukunishi, S.; Tachikawa, T. Polysilane-inserted methylammonium lead iodide perovskite solar cells doped with formamidinium and potassium. Energies 2020, 13, 4776. [Google Scholar] [CrossRef]
- Tong, J.; Song, Z.; Kim, D.M.; Chen, X.; Chen, C.; Palmstrom, A.F.; Ndione, P.F.; Reese, M.O.; Dunfield, S.P.; Reid, O.G.; et al. Carrier lifetimes of >1 μs in Sn-Pb perovskites enable efficient all-perovskite tandem solar cells. Science 2019, 364, 475–479. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.K.; Zhuo, M.P.; Hu, Y.; Hu, K.H.; Ye, Q.Q.; Jain, S.M.; Yang, Y.G.; Gao, X.Y.; Liao, L.S. Pb−Sn−Cu ternary organometallic halide perovskite solar cells. Adv. Mater. 2018, 30, 1800258. [Google Scholar] [CrossRef]
- Oku, T.; Ohishi, Y.; Suzuki, A. Effects of antimony addition to perovskite-type CH3NH3PbI3 photovoltaic devices. Chem. Lett. 2016, 45, 134–136. [Google Scholar] [CrossRef]
- Oku, T.; Ohishi, Y.; Suzuki, A.; Miyazawa, Y. Effects of Cl addition to Sb-doped perovskite-type CH3NH3PbI3 photovoltaic devices. Metals 2016, 6, 147. [Google Scholar] [CrossRef]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Oku, T.; Suzuki, K.; Suzuki, A. Effects of chlorine addition to perovskite-type CH3NH3PbI3 photovoltaic devices. J. Ceram. Soc. Jpn. 2016, 124, 234–238. [Google Scholar] [CrossRef]
- Oku, T.; Ohishi, Y.; Suzuki, A.; Miyazawa, Y. Effects of NH4Cl addition to perovskite CH3NH3PbI3 photovoltaic devices. J. Ceram. Soc. Jpn. 2017, 125, 303–307. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T.; Ohishi, Y.; Tanaka, H.; Suzuki, A. Effects of excess PbI2 addition to CH3NH3PbI3−xClx perovskite solar cells. Chem. Lett. 2018, 47, 528–531. [Google Scholar] [CrossRef]
- Oku, T.; Ohishi, Y. Effects of annealing on CH3NH3PbI3(Cl) perovskite photovoltaic devices. J. Ceram. Soc. Jpn. 2018, 126, 56–60. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T.; Suzuki, A.; Sakamoto, H.; Yamada, M.; Minami, S.; Miyauchi, S. Fabrication and characterization of CH3NH3(Cs)Pb(Sn)I3(Cl) perovskite solar cells with TiO2 nanoparticle layers. Jpn. J. Appl. Phys. 2018, 57, 02CE03. [Google Scholar] [CrossRef]
- Taguchi, M.; Suzuki, A.; Oku, T.; Ueoka, N.; Minami, S.; Okita, M. Effects of annealing temperature on decaphenylcyclopentasilane-inserted CH3NH3PbI3 perovskite solar cells. Chem. Phys. Lett. 2019, 737, 136822. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T.; Tanaka, H.; Suzuki, A.; Sakamoto, H.; Yamada, M.; Minami, S.; Miyauchi, S.; Tsukada, S. Effects of PbI2 addition and TiO2 electron transport layers for perovskite solar cells. Jpn. J. Appl. Phys. 2018, 57, 08RE05. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T. Stability characterization of PbI2-added CH3NH3PbI3−xClx photovoltaic devices. ACS Appl. Mater. Interfaces 2018, 10, 44443–44451. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T.; Suzuki, A. Additive effects of alkali metals on Cu-modified CH3NH3PbI3−δClδ photovoltaic devices. RSC Adv. 2019, 9, 24231–24240. [Google Scholar] [CrossRef]
- Cortecchia, D.; Dewi, H.A.; Yin, J.; Bruno, A.; Chen, S.; Baikie, T.; Boix, P.P.; Grätzel, M.; Mhaisalkar, S.; Soci, C.; et al. Lead-free MA2CuClxBr4−x hybrid perovskites. Inorg. Chem. 2016, 55, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Ueoka, N.; Oku, T. Effects of co-addition of sodium chloride and copper(II) bromide to mixed-cation mixed-halide perovskite photovoltaic devices. ACS Appl. Energy Mater. 2020, 3, 7272–7283. [Google Scholar] [CrossRef]
- Oku, T.; Zushi, M.; Imanishi, Y.; Suzuki, A.; Suzuki, K. Microstructures and photovoltaic properties of perovskite-type CH3NH3PbI3 compounds. Appl. Phys. Express 2014, 7, 121601. [Google Scholar] [CrossRef]
- Oku, T.; Ohishi, Y.; Ueoka, N. Highly (100)-oriented CH3NH3PbI3(Cl) perovskite solar cells prepared with NH4Cl using an air blow method. RSC Adv. 2018, 8, 10389–10395. [Google Scholar] [CrossRef] [PubMed]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T.; Suzuki, A. Effects of doping with Na, K, Rb, and formamidinium cations on (CH3NH3)0.99Rb0.01Pb0.99Cu0.01I3−x(Cl, Br)x perovskite photovoltaic cells. AIP Adv. 2020, 10, 125023. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).