Synthesis, Characterization, and Functionalization of Chitosan and Gelatin Type B Nanoparticles to Develop Novel Highly Biocompatible Cell-Penetrating Agents †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Type II Gelatin and Chitosan Nanoparticles
2.3. Functionalization of Nanoparticles with Buforin II
2.4. Labeling of NPs-BUF-II-Nanobioconjugates with Rhodamine B
2.5. Characterization
3. Results and Discussion
3.1. Size Distribution and Surface Charge (Z Potential)
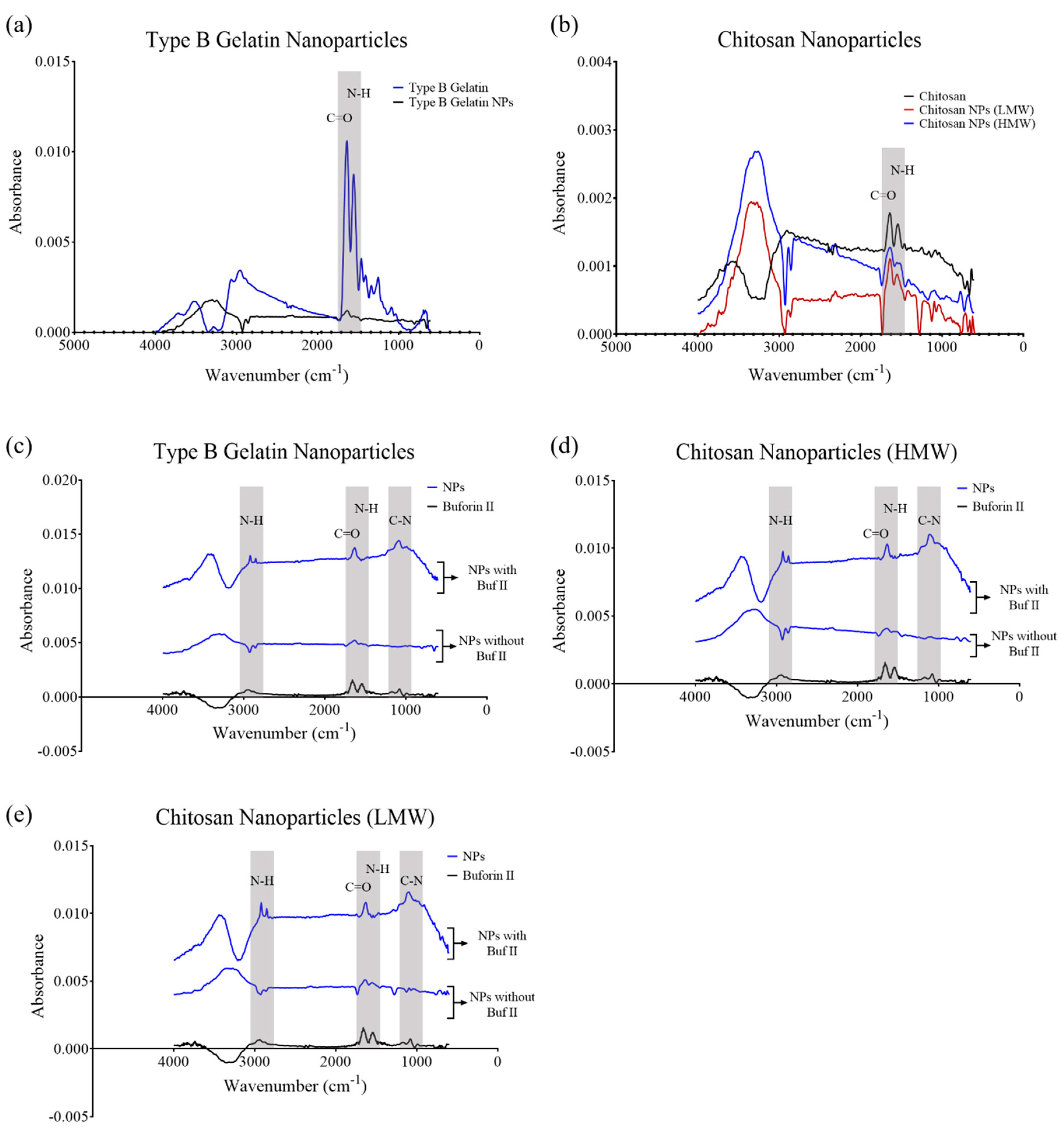
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
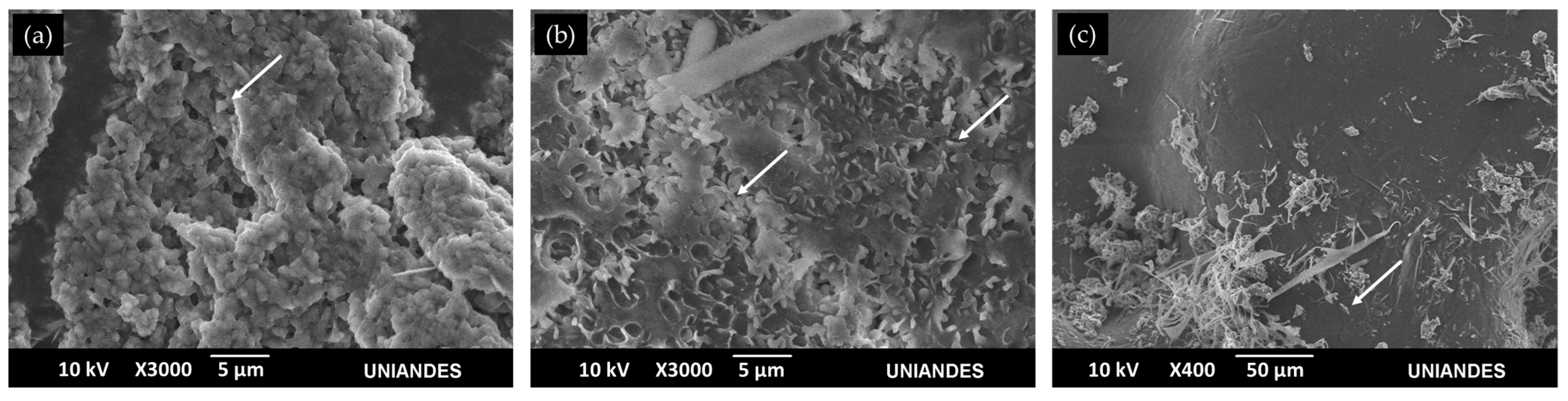
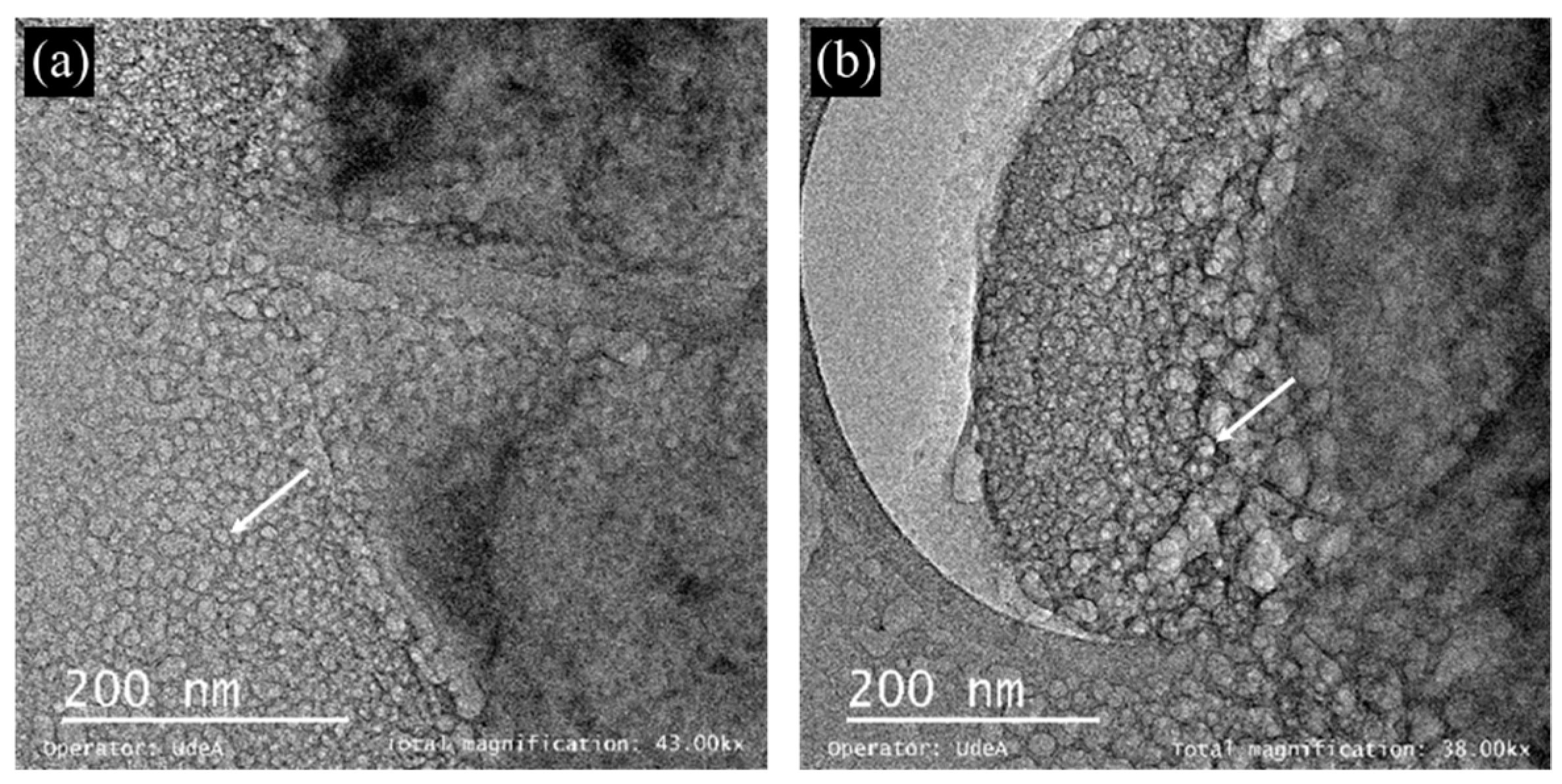
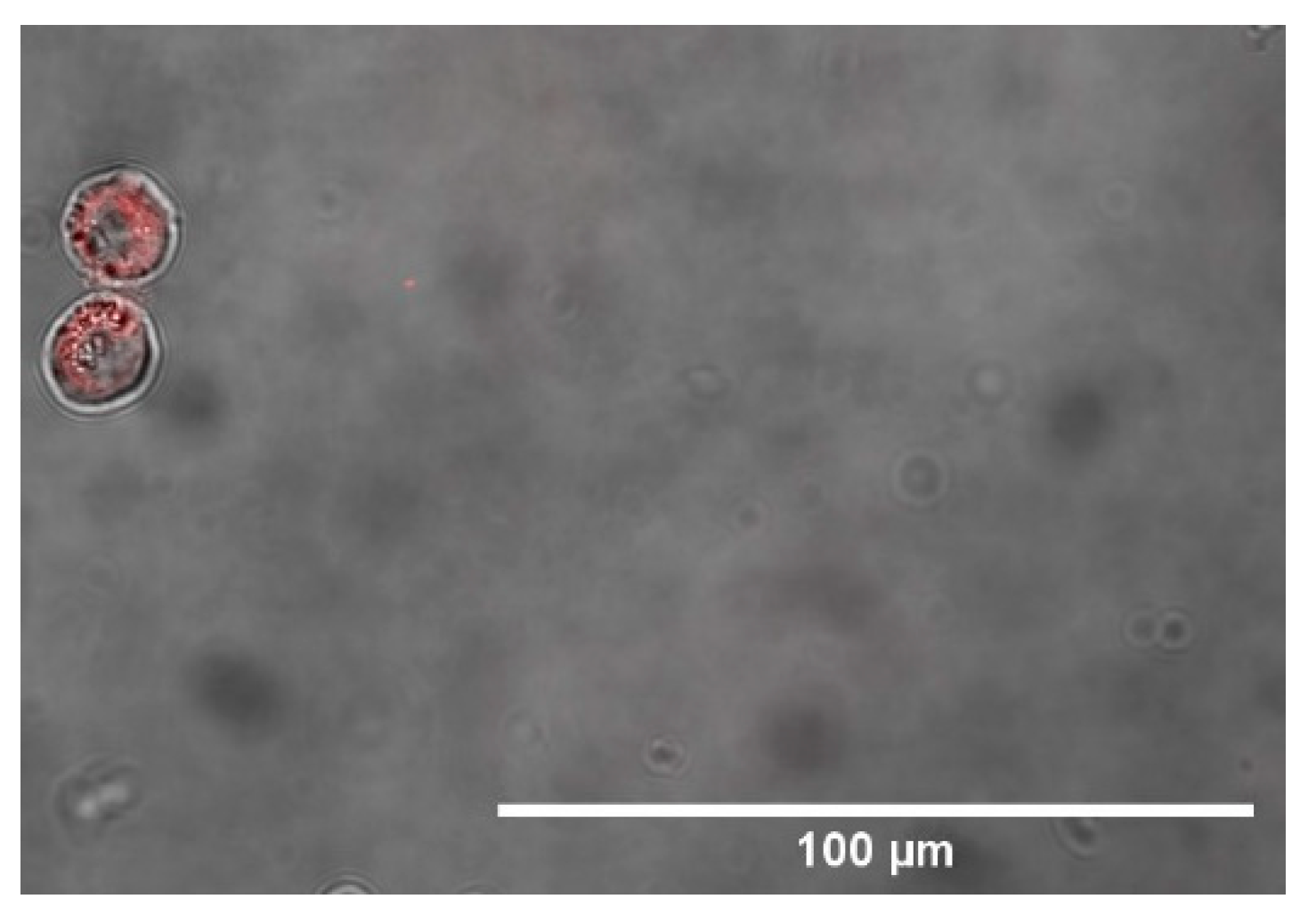
3.3. Microscope Imaging (SEM, TEM and Confocal)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arms, L.; Smith, D.W.; Flynn, J.; Palmer, W.; Martin, A.; Woldu, A.; Hua, S. Advantages and limitations of current techniques for analyzing the biodistribution of nanoparticles. Front. Pharmacol. 2018, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Jumeaux, C.; Gupta, B.; Stevens, M.M. Peptide and protein nanoparticle conjugates: Versatile platforms for biomedical applications. Chem. Soc. Rev. 2018, 47, 3574–3620. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Bennet, D.; Kim, S. Polymer Nanoparticles for Smart Drug Delivery. In Application of Nanotechnology in Drug Delivery; InTech: London, UK, 2014. [Google Scholar]
- Wang, W.; Gaus, K.; Tilley, R.D.; Gooding, J.J. The impact of nanoparticle shape on cellular internalisation and transport: What do the different analysis methods tell us? Mater. Horizons 2019, 6, 1538–1547. [Google Scholar] [CrossRef]
- Sharma, D.; Sharma, N.; Pathak, M.; Agrawala, P.K.; Basu, M.; Ojha, H. Nanotechnology-based drug delivery systems: Challenges and opportunities. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Elsevier: Amsterdam, Netherlands, 2018; pp. 39–79. ISBN 9780128136898. [Google Scholar]
- Selby, L.I.; Cortez-Jugo, C.M.; Such, G.K.; Johnston, A.P.R. Nanoescapology: Progress toward understanding the endosomal escape of polymeric nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2017, 9. [Google Scholar] [CrossRef]
- Ramírez-Acosta, C.M.; Cifuentes, J.; Cruz, J.C.; Reyes, L.H. Patchy Core/Shell, Magnetite/Silver Nanoparticles via Green and Facile Synthesis: Routes to Assure Biocompatibility. Nanomaterials 2020, 10, 1857. [Google Scholar] [CrossRef]
- Cuellar, M.; Cifuentes, J.; Perez, J.; Suarez-Arnedo, A.; Serna, J.; Groot, H.; Muñoz-Camargo, C.; Cruz, J. Novel BUF2-magnetite nanobioconjugates with cell-penetrating abilities. Int. J. Nanomed. 2018, 13, 8087–8094. [Google Scholar] [CrossRef]
- Perez, J.; Rueda, J.; Cuellar, M.; Suarez-Arnedo, A.; Cruz, J.C.; Muñoz-Camargo, C. Cell-Penetrating And Antibacterial BUF-II Nanobioconjugates: Enhanced Potency Via Immobilization On Polyetheramine-Modified Magnetite Nanoparticles. Int. J. Nanomed. 2019, 14, 8483–8497. [Google Scholar] [CrossRef]
- Lopez-Barbosa, N.; Suárez-Arnedo, A.; Cifuentes, J.; Gonzalez Barrios, A.F.; Silvera Batista, C.A.; Osma, J.F.; Munõz-Camargo, C.; Cruz, J.C. Magnetite-OmpA Nanobioconjugates as Cell-Penetrating Vehicles with Endosomal Escape Abilities. ACS Biomater. Sci. Eng. 2020, 6, 415–424. [Google Scholar] [CrossRef]
- Lopez-Barbosa, N.; Garcia, J.G.; Cifuentes, J.; Castro, L.M.; Vargas, F.; Ostos, C.; Cardona-Gomez, G.P.; Hernandez, A.M.; Cruz, J.C. Multifunctional magnetite nanoparticles to enable delivery of siRNA for the potential treatment of Alzheimer’s. Drug Deliv. 2020, 27, 864–875. [Google Scholar] [CrossRef]
- Ramírez-Acosta, C.M.; Cifuentes, J.; Castellanos, M.C.; Moreno, R.J.; Muñoz-Camargo, C.; Cruz, J.C.; Reyes, L.H. PH-Responsive, Cell-Penetrating, Core/Shell Magnetite/Silver Nanoparticles for the Delivery of Plasmids: Preparation, Characterization, and Preliminary In Vitro Evaluation. Pharmaceutics 2020, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Cervia, L.D.; Chang, C.C.; Wang, L.; Yuan, F. Distinct effects of endosomal escape and inhibition of endosomal trafficking on gene delivery via electrotransfection. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Dana, H.; Chalbatani, G.M.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular Mechanisms and Biological Functions of siRNA. Int. J. Biomed. Sci. 2017, 13, 48–57. [Google Scholar] [PubMed]
- Smith, S.A.; Selby, L.I.; Johnston, A.P.R.; Such, G.K. The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery. Bioconjug. Chem. 2019, 30, 263–272. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Jafari, S.; Khosroushahi, A.Y. A sight on the current nanoparticle-based gene delivery vectors. Nanoscale Res. Lett. 2014, 9, 1–9. [Google Scholar] [CrossRef]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Producing gelatin nanoparticles as delivery system for bovine serum albumin. Iran. Biomed. J. 2013, 18, 34–40. [Google Scholar] [CrossRef]
- Carmona, E.R.; Plaza, T.; Recio-Sánchez, G.; Parodi, J. Generation of a protocol for the synthesis of chitosan nanoparticles loaded with florfenicol through the ionic gelation method. Rev. Investig. Vet. del Perú 2018, 29, 1195–1202. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation of rhodamine B and methyl orange over boron-doped g-C 3N4 under visible light irradiation. Langmuir 2010, 26, 3894–3901. [Google Scholar] [CrossRef]
- ImageJ. Available online: https://imagej.net/Welcome (accessed on 19 April 2021).
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13. [Google Scholar] [CrossRef]
- Kommareddy, S.; Shenoy, D.B.; Amiji, M.M. Gelatin Nanoparticles and Their Biofunctionalization. In Nanotechnologies for the Life Sciences; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Ahsan, S.M.; Rao, C.M. The role of surface charge in the desolvation process of gelatin: Implications in nanoparticle synthesis and modulation of drug release. Int. J. Nanomed. 2017, 12, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A. Taranjit Preparation of chitosan nanoparticles: A study of influencing factors. In Proceedings of the AIP Conference Proceedings, Chandigarh, India, 23–26 February 2011; Volume 1393, pp. 299–300. [Google Scholar]
- Nangia, S.; Sureshkumar, R. Effects of nanoparticle charge and shape anisotropy on translocation through cell membranes. Langmuir 2012, 28, 17666–17671. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, C.; Reyes, L.H.; Muñoz-Camargo, C.; Cruz, J.C. Synthesis, Characterization, and Functionalization of Chitosan and Gelatin Type B Nanoparticles to Develop Novel Highly Biocompatible Cell-Penetrating Agents. Mater. Proc. 2021, 4, 30. https://doi.org/10.3390/IOCN2020-07816
González C, Reyes LH, Muñoz-Camargo C, Cruz JC. Synthesis, Characterization, and Functionalization of Chitosan and Gelatin Type B Nanoparticles to Develop Novel Highly Biocompatible Cell-Penetrating Agents. Materials Proceedings. 2021; 4(1):30. https://doi.org/10.3390/IOCN2020-07816
Chicago/Turabian StyleGonzález, Cristina, Luis H. Reyes, Carolina Muñoz-Camargo, and Juan C. Cruz. 2021. "Synthesis, Characterization, and Functionalization of Chitosan and Gelatin Type B Nanoparticles to Develop Novel Highly Biocompatible Cell-Penetrating Agents" Materials Proceedings 4, no. 1: 30. https://doi.org/10.3390/IOCN2020-07816
APA StyleGonzález, C., Reyes, L. H., Muñoz-Camargo, C., & Cruz, J. C. (2021). Synthesis, Characterization, and Functionalization of Chitosan and Gelatin Type B Nanoparticles to Develop Novel Highly Biocompatible Cell-Penetrating Agents. Materials Proceedings, 4(1), 30. https://doi.org/10.3390/IOCN2020-07816







