1. Introduction
It is understood that high amounts of dissolved Al in steel is likely to react with less stable oxides such as SiO2 in CaO-SiO2 based mould fluxes. As a consequence of this reaction, Si enters the liquid steel and Al2O3 enters the molten fluxes. The change in the mould flux composition generally leads to variations in the slag viscosity during the casting process and this change in viscosity deteriorates the casting process and declines the quality of the slab surface. Being one of the most consequential properties of metallurgical slags, viscosity is pertinent to the composition of the mould flux and the temperature. Thus, an investigation into the effects of compositional change on mould flux properties will be beneficial for the design of less reactive mould fluxes.
There has been much research done by scholars looking into the effect of the CaO/SiO
2 ratio and Al
2O
3/SiO
2 ratio on the properties of lime-silica based mould fluxes to inhibit the sudden change in viscosity. However, it is also widely agreed that it is not easy to stabilise the viscosity of lime-silica based mould fluxes because of the reaction between the molten steel and slag [
1]. One suggestion has been that the determination of the optimal CaO/Al
2O
3 ratio holds a significant role in the design of mould fluxes for high-Al steel casting [
2,
3]. Yan et al. [
4] systematically studied the consequence of the CaO/Al
2O
3 ratio on the viscosity of lime-alumina based mould fluxes by making use of the rotational cylinder method and suggesting that as the CaO/Al
2O
3 ratio ranged from 0.6 to 3.2, the viscosity first decreased and then increased below 1270 °C. In addition, Blazek et al. [
5] revealed that the viscosity of the mould flux decreased with an increase in the CaO/Al
2O
3 ratio. A widely accepted approach for the development of lime-alumina based mould fluxes is that the CaO/Al
2O
3 ratio (C/A ratio) has an important effect on slag crystallisation. Therefore, it is important to determine the optimal C/A ratio by looking into the effects of compositional change on slag properties. There are several studies [
4,
6,
7] that have taken a closer look into the effects of the C/A ratio on the thermo-physical properties of lime-alumina based mould fluxes. These studies mainly fixate on the viscosity of the mould flux with small changes in the C/A ratio. Hence, it is necessary to investigate the effect of a broad range of C/A ratios on thermo-physical properties of lime-alumina based mould fluxes.
3. Result and Discussion
The Inclined Plane Test (IPT) and FactSage software were used to investigate the effect of B
2O
3 addition and C/A ratio on viscosity behaviour of the P, C and T series mould fluxes, as can be seen listed in
Table 1. In
Table 2, the results of the viscosity measurements derived by using both techniques for the P and T series lime-alumina based mould fluxes can be found. The Inclined Plane Test (IPT) could not be applied to the samples of P1, P2, P4 and P5 in the same way, as their viscosities are too high at 1300 °C, and so the test becomes invalid.
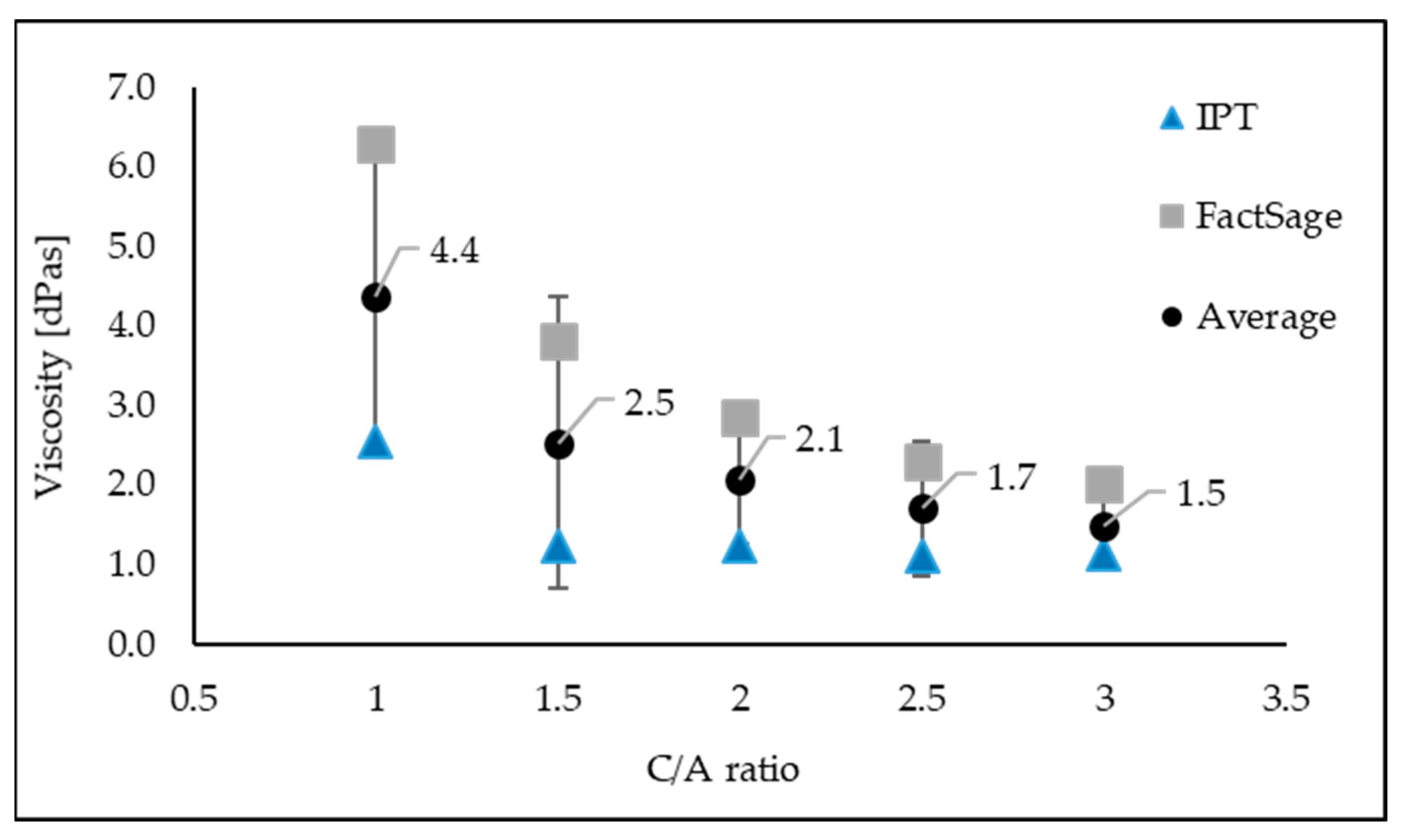
As can be seen from
Table 2, the viscosity decreases from 3.18 to 1.06 poise with an increase in B
2O
3 at a fixed C/A ratio of 1.2. The calculated viscosities from the FactSage software for these two mould fluxes also present us with the same trend within the experimental results, however, the values are noticeably different. The reason behind this difference may be caused by the limited thermodynamic data references for the lime-alumina based slag system in the FactSage software package.
Upon close inspection of
Figure 2, the change in the mould flux viscosity as a function of the C/A ratio at 1300 °C is clearly demonstrated.
Figure 2 clearly shows that the viscosity of the mould fluxes decreases rapidly when the C/A ratio is changed from 1 to 1.5. The viscosity of the C-series mould fluxes clearly demonstrates that with an increase in the C/A ratio ranging from 1.5 to 2.5, the viscosity slightly decreases, although when the C/A ratio surpasses 2.5, the viscosity nearly levels off.
Figure 2 also brings to light the comparison of the experimental data acquired by the IPT with the estimations of the viscosities by FactSage. The viscosity of C-series mould fluxes acquired by these particular methods presents the same bearing. Additionally, both the measured and estimated viscosities decrease with an increase in the C/A ratio. The methods of measurement for viscosity used in this study were credible when the experimental uncertainties associated with the viscosity determination tests were considered. Nonetheless, a good correlation between the measured and the calculated viscosities was observed.
It is general knowledge that Al
2O
3 is an amphoteric oxide and has an exceptional effect on the viscosity of mould fluxes. It behaves as a basic oxide (network modifier) or an acidic oxide (network former) depending on the basicity of the slag. The literature extensively details the nature of alumina in the melt. In the lime-alumina melts, Al-O complexes present 4-, 5- and 6-fold coordinations and form as AlO
4, AlO
5 and AlO
6 units [
9,
10].
Additionally, at higher temperatures, more alumina is needed in the formation of the aluminate structure with a decrease in the C/A ratio. This causes an increase in the degree of polymerisation as well as an increase in the viscosity of the melt. Be that it may, with an increase in the C/A ratio, more O
2- ions are able to depolymerise the aluminate network and ergo reduce the viscosity of the melt [
4,
11,
12]. At lower temperatures, particles with higher melting temperatures begin to precipitate in the melt and these solid particles start to control the viscosity. Consequently, the viscosity of the mould flux increases.
The mole fraction of basic oxides (CaO and Na
2O) exceeds the mole fraction of Al
2O
3 for the fluxes used in this part of the study. Consequently, alumina operates as an acidic oxide and has the capacity to promote [AlO
4]
5- ions when an adequate charge balance is available by basic oxides. Any remaining excess Ca
2+ cations in the CaO-Al
2O
3 melt depolymerise AlO
4 structural units and lessen the viscosity. Thus, in the C-series mould fluxes, whilst the viscosity decreases, the C/A ratio increases. As reflected in
Figure 2, the viscosity abruptly decreases followed by it gradually becoming stable with an increase in yhe C/A ratio. This is because the further increase in the C/A ratio supplies an excess of free O
2- ions, and hence the viscosity ultimately becomes stable.
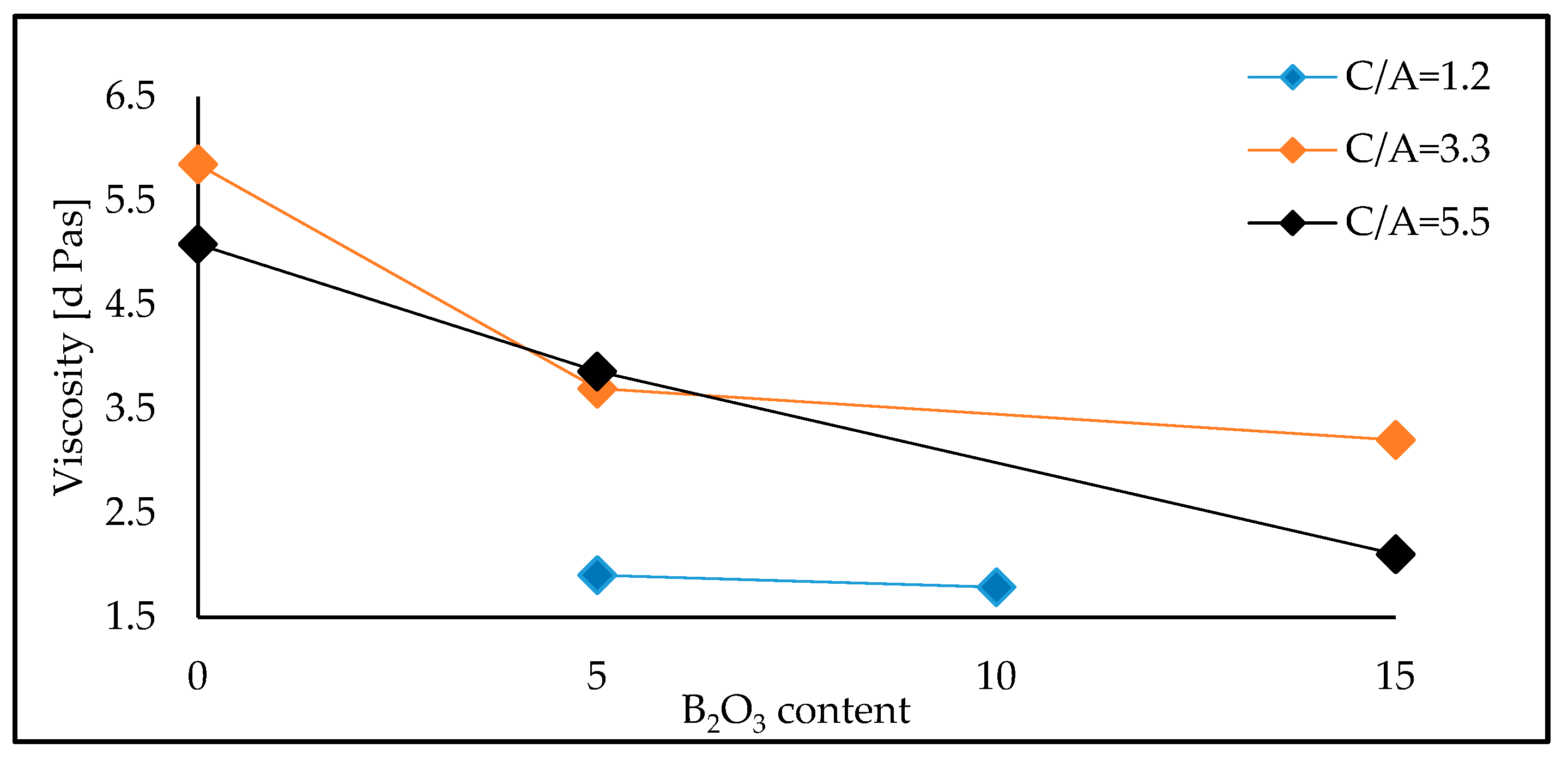
Figure 3 demonstrates the variation in viscosity of the investigated mould fluxes with the addition of B
2O
3 at a temperature of 1300 °C using the FactSage software—where the B
2O
3 content was 0, 5, 10 and 15 wt.% at each C/A ratio of 1.2, 3.3 and 5.5. The viscosity of the mould fluxes is reduced with the addition of B
2O
3 at all three of the studied C/A ratios. However, it is also particularly noticeable that the measured viscosity decreases when B
2O
3 is added regardless of the C/A ratio at a temperature of 1300 °C. This is seen to be consistent with the reported results [
12,
13,
14,
15]. Huang et al. [
13] looked into the effect of adding 0, 3, 6 and 9 wt.% B
2O
3 on the viscosity of mould fluxes containing a low silica content. It was pointed out that the viscosity decreased with an increasing B
2O
3 content. The results of this study suggest that adding B
2O
3 substantially decreases the melting temperature of the slag, and therefore increases the degree of superheat in the melt. Under higher degrees of superheat, the mobility of the particles in the melt rises and thus the viscosity decreases. The authors also bring to attention that boron is usually 3- or 4- coordinated with oxygen and that the addition of CaO or Na
2O, may cause configuration changes in the melt [
11,
13]. Since the mould flux in the work of Kim et al [
12] contains more than 50% network modifiers, the BO
3 is dominant in these melts. Additionally, according to Fourier Transform Infra-Red (FTIR) spectroscopy results carried out in this work, the amount of 3-coordinated boron is higher than 4- coordinated boron in the slag samples. This tells us that when adding B
2O
3, charge compensating cations break the B-O bonds and BO
3- units form in the melt with additional non-bridging oxygen. Therefore, the structure becomes simpler and thus the viscosity decreases.
The measured viscosity of the mould fluxes with a constant amount of 15 wt.% B
2O
3 is more or less stable when the C/A ratio increases from 3.3 to 5.5. The FactSage viscosity calculations for P3 and P6 demonstrate a decreasing trend that holds no similarities to the experimental results as the absolute values are higher than the experimental results. Lately, some researchers have suggested [
4,
12,
15] that adding B
2O
3 has a somewhat minor effect on the slag viscosity when the mould flux has a high C/A ratio. Research done by Kim and Sohn [
12] brought to light that increasing the C/A ratio for mould fluxes containing 18 wt.% B
2O
3 beyond a C/A ratio of 1 had a minor effect on viscosity. A likely explanation derived from the authors’ work suggested that the existing B
2O
3 and Na
2O modified the slag structure and caused the disappearance of large complex structures in the melt. For this reason, increasing the C/A ratio will supply free oxygen ions to the melt and so the viscosity will stabilise.
5 wt.% Li
2O and B
2O
3 at various percentages were added to the P-series mould fluxes. Yu et al. [
15] researched the viscosity of mould fluxes for the casting of high-Al TRIP steels and made the suggestion that adding Li
2O and B
2O
3 to mould fluxes could have a synergistic effect in stabilising the acidity as well as stabilising the amphoteric characteristics of Al
2O
3 and that, as a result, the viscosity should level out. This also correlates with the research done by Omoto et al. [
16].
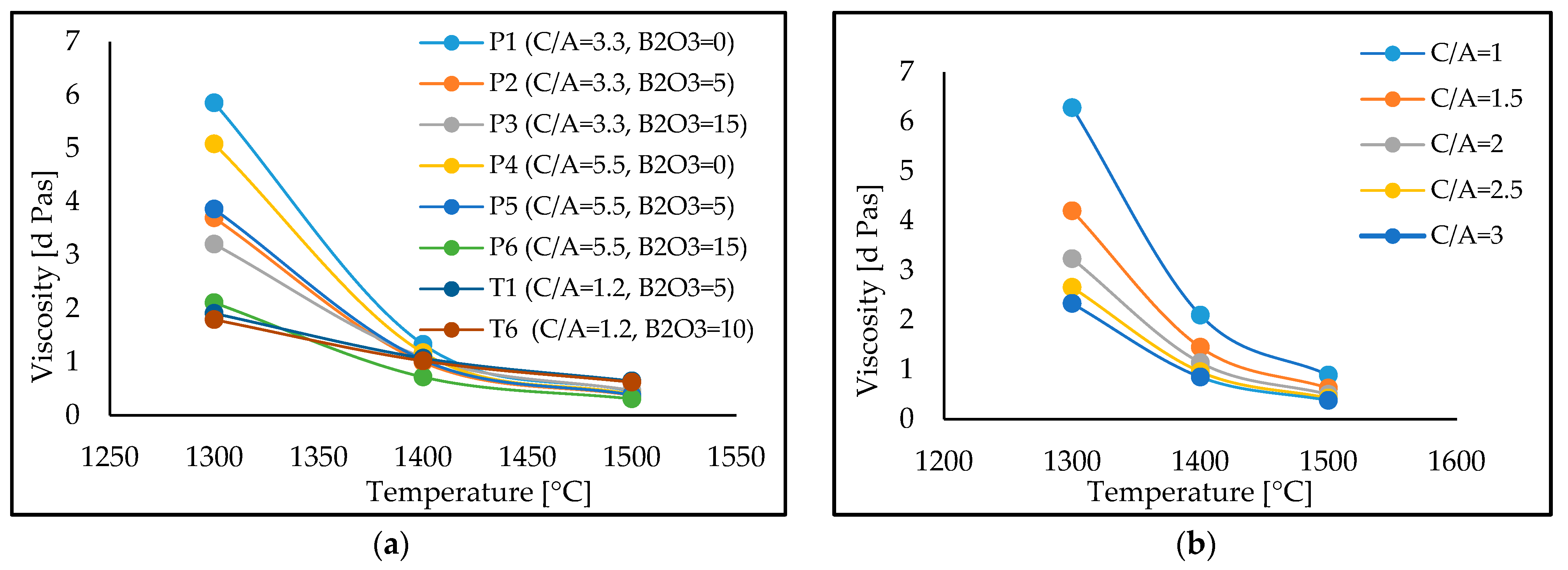
The effect that temperature has on the viscosity of the lime-alumina based mould fluxes with different C/A ratios and B
2O
3 contents are shown in
Figure 4, where the viscosity is seen to be decreasing with an increasing temperature at all C/A ratios, as expected. The calculated viscosity decreases with an increasing B
2O
3 content at C/A ratios of 1.2, 3.3 and 5.5 at varying temperatures. Nonetheless, at temperatures of 1300 °C, 1400 °C and 1500 °C, the viscosity of the C-series mould fluxes decreases with an increase in the C/A ratio.
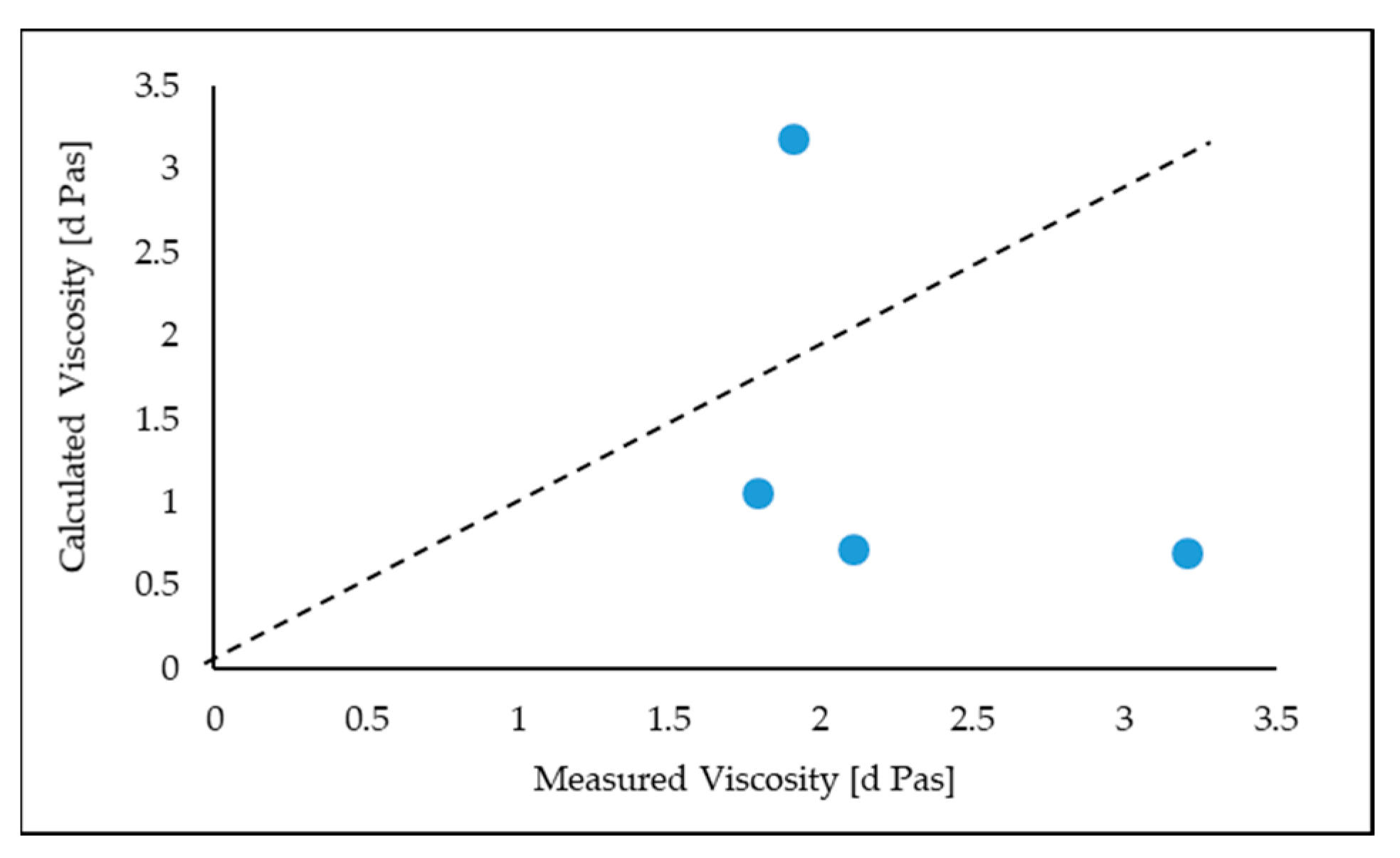
Figure 5 demonstrates the comparison of experimental data obtained by the IPT (Inclined Plane Test) with the viscosities estimated by the FactSage software. As reflected in
Figure 5, the agreements between the calculated viscosities from FactSage and the measured values are unreasonable even though the calculated viscosities display the same trend as the measured viscosities for an increase in B
2O
3 content.
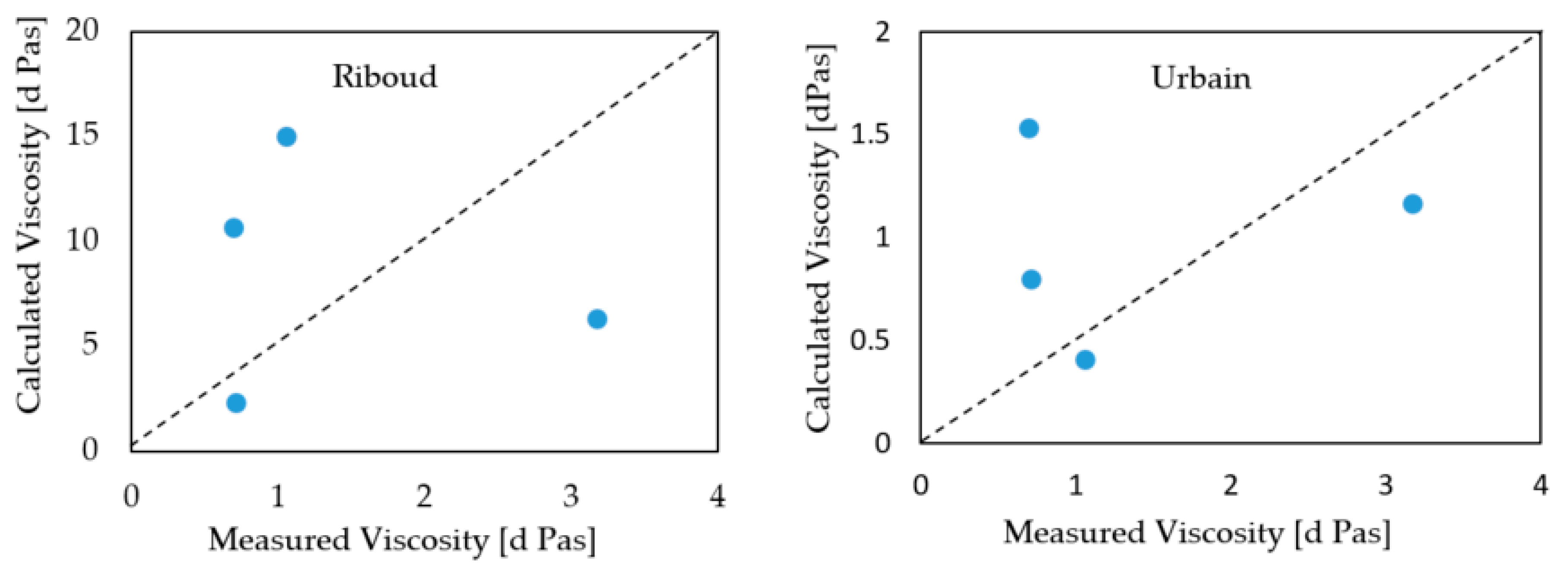
The calculations regarding the viscosities of P-series, T1 and T6 lime-alumina based mould fluxes have also been made using the Riboud and Urbain models.
Figure 6 displays a comparison of the measured viscosities and data calculated from the Urbain and Riboud models. The correspondence between the estimated viscosities from the Urbain model and experimental results are reasonably good at the C/A ratio of 1.2. At the fixed C/A ratios of 3.3 and 5.5, there is a considerable deviation between the experimental data and calculated viscosities from both the Urbain and Riboud models. As reported by the viscosities estimated by FactSage, the viscosity of the P-series mould fluxes is seen to decrease with additions of B
2O
3. The estimated viscosities from both the Urbain and Riboud models present opposite trends to the viscosities estimated by the FactSage software, when regarding the effect of B
2O
3 addition.
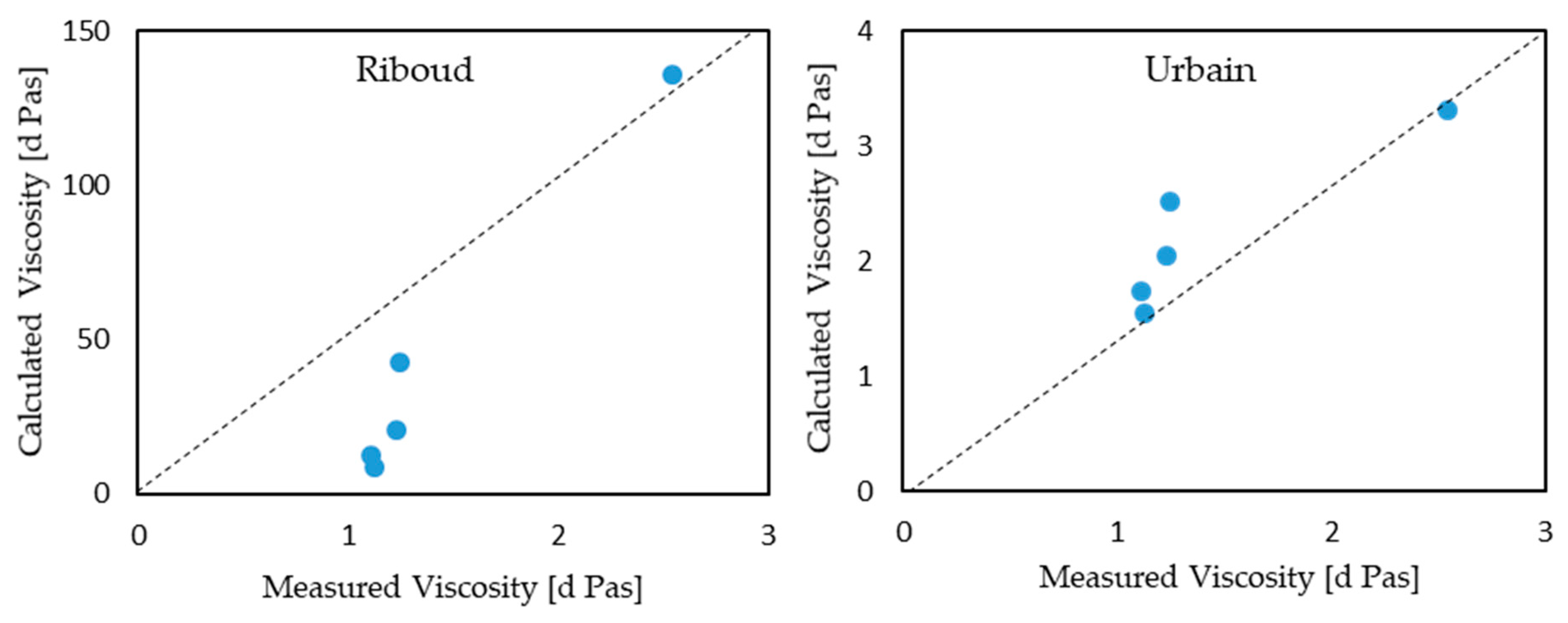
Riboud and Urbain models have also been used to calculate the viscosities of the C-series lime-alumina based mould fluxes.
Figure 7 displays the comparison of the viscosities measured as well as the data calculated by the Urbain and Riboud models. Although both models present a similar trend (ie. the viscosity of the mould flux decreases with an increase in C/A ratio) with the viscosities measured by the inclined plane test, the calculated viscosities using the Riboud model are considerably higher in contrast to the measured results. Nonetheless, the Urbain viscosity prediction model presents a relatively better estimation. The cause behind this large discrepancy in the Riboud model can be explained due to B
2O
3 being classified as a basic oxide in the Riboud model while it was treated as an amphoteric oxide in the Urbain model.