Synthesis, Characterization, and Functionalization of Graphene Oxide-Based Nanoplatforms for Gene Delivery †
Abstract
:1. Introduction
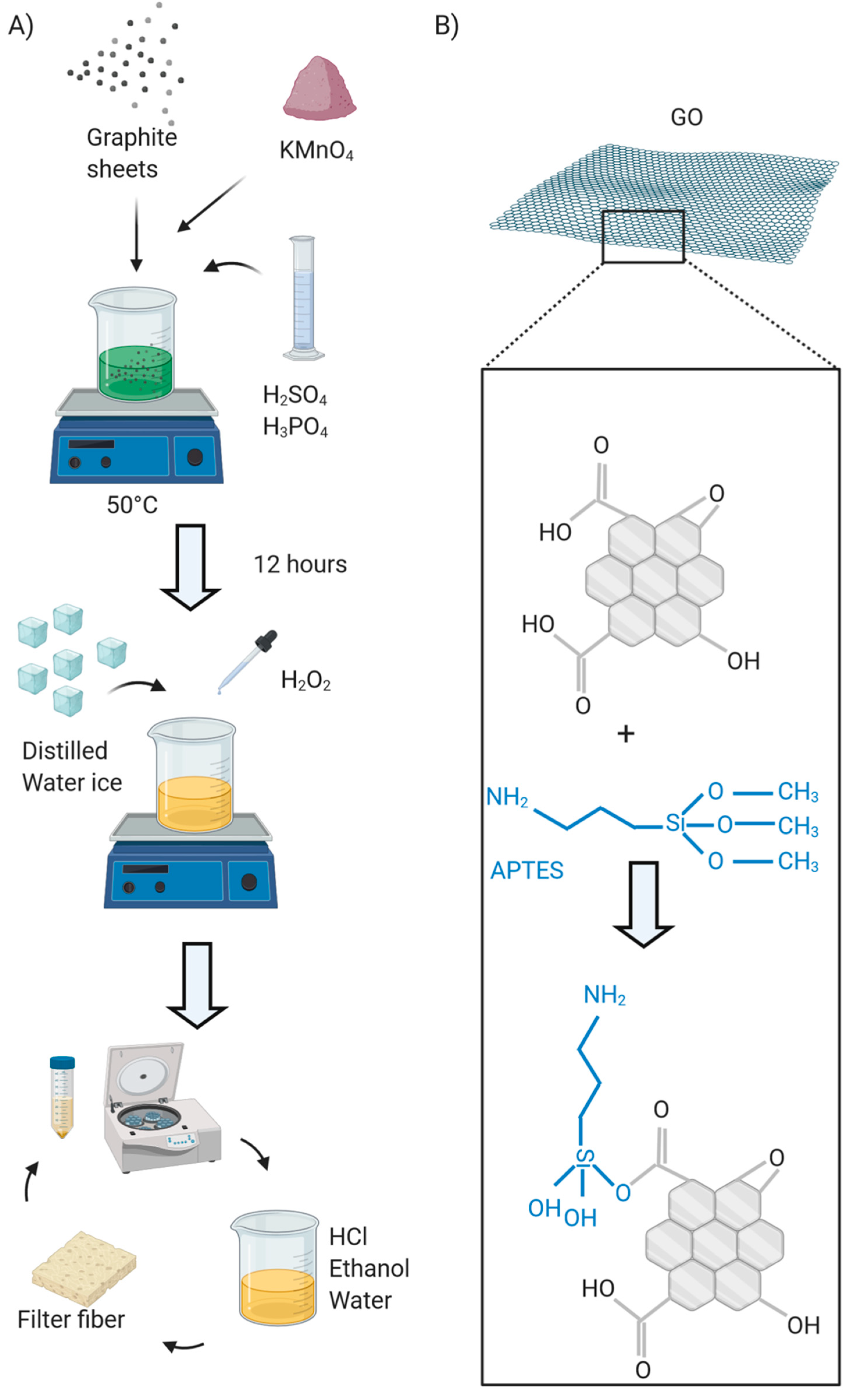
2. Materials and Methods
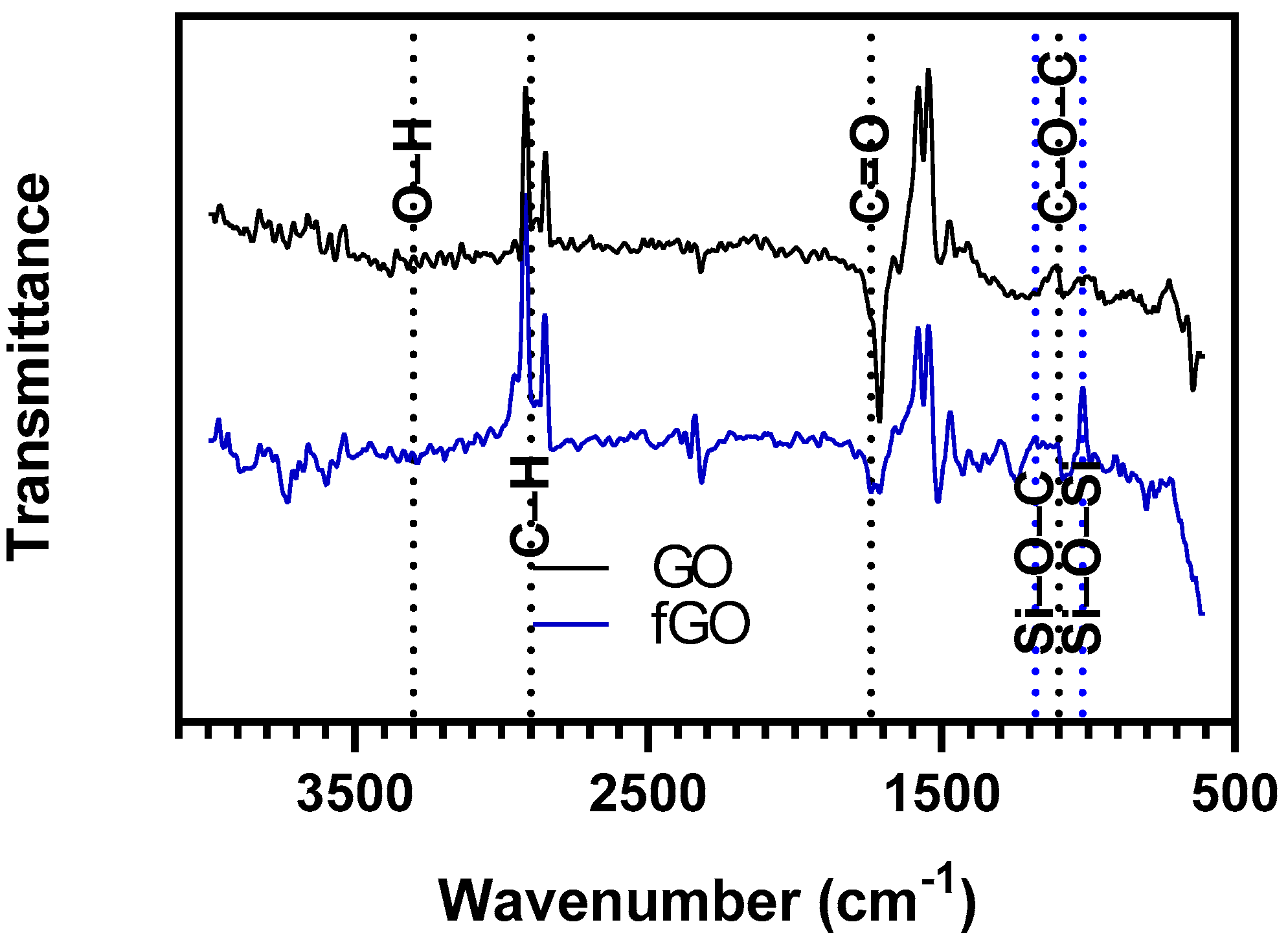
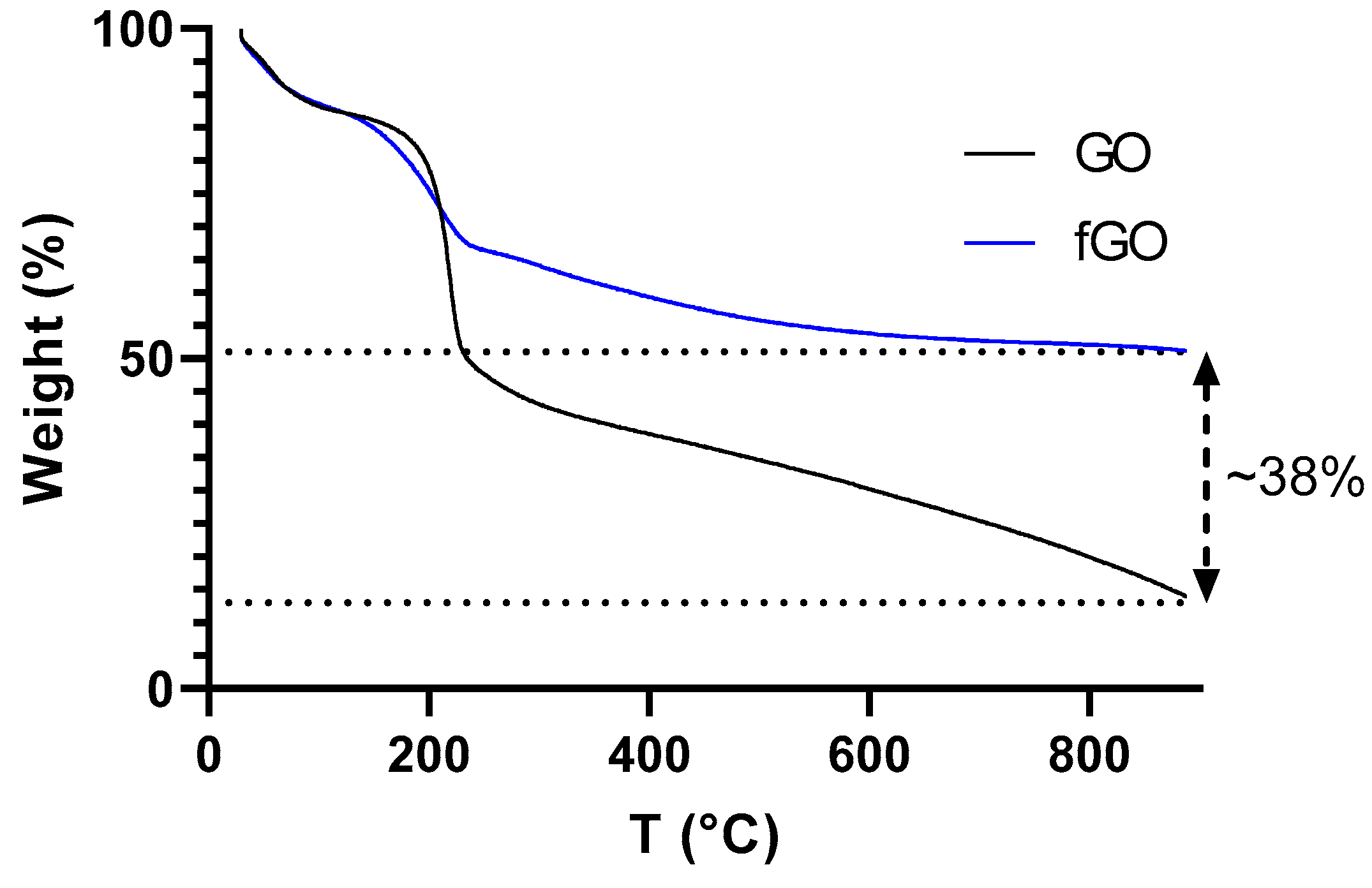
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nayerossadat, N.; Ali, P.A.; Maedeh, T. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, D.; Elaissari, A.; Fessi, H. Gene therapy and DNA delivery systems. Int. J. Pharm. 2014, 459, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Ebara, M.; Uto, K. Gold Nanomaterials for Gene Therapy; Elsevier Ltd.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Mady, M.M. Cationic liposomes as gene delivery system. Afr. J. Pharm. Pharmacol. 2011, 5, 2007–2012. [Google Scholar]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Malmsten, M. Inorganic nanomaterials as delivery systems for proteins, peptides, DNA, and siRNA. Curr. Opin. Colloid Interface Sci. 2013, 18, 468–480. [Google Scholar] [CrossRef]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, M.; Cifuentes, J.; Perez, J.; Suarez-Arnedo, A.; Serna, J.A.; Groot, H.; Muñoz-Camargo, C.; Cruz, J.C. Novel BUF2-magnetite nanobioconjugates with cell-penetrating abilities. Int. J. Nanomed. 2018, 13, 8087–8094. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Gu, B.; Lin, Y.; Panwar, N.; Tjin, S.C.; Qu, J.; Lau, S.P.; Yong, K.-T. Functionalized 2D nanomaterials for gene delivery applications. Coord. Chem. Rev. 2017, 347, 77–97. [Google Scholar] [CrossRef]
- Rahmanian, N.; Eskandani, M.; Barar, J.; Omidi, Y. Recent trends in targeted therapy of cancer using graphene oxide-modified multifunctional nanomedicines. J. Drug Target. 2016, 25, 202–215. [Google Scholar] [CrossRef] [PubMed]
- De Melo-Diogo, D.; Lima-Sousa, R.; Alves, C.G.; Costa, E.C.; Louro, R.O.; Correia, I.J. Functionalization of graphene family nanomaterials for application in cancer therapy. Colloids Surf. B Biointerfaces 2018, 171, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, S.; Liu, X.; Ding, J.; Zhou, W. Platinated graphene oxide: A nanoplatform for efficient gene-chemo combination cancer therapy. Eur. J. Pharm. Sci. 2018, 121, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Zawisza, B.; Baranik, A.; Malicka, E.; Talik, E.; Sitko, R. Preconcentration of Fe(III), Co(II), Ni(II), Cu(II), Zn(II) and Pb(II) with ethylenediamine-modified graphene oxide. Microchim. Acta 2016, 183, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Bouazizi, N.; Vieillard, J.; Bargougui, R.; Couvrat, N.; Thoumire, O.; Morin, S.; Ladam, G.; Mofaddel, N.; Brun, N.; Azzouz, A.; et al. Entrapment and stabilization of iron nanoparticles within APTES modified graphene oxide sheets for catalytic activity improvement. J. Alloys Compd. 2019, 771, 1090–1102. [Google Scholar] [CrossRef]
- Leaper, S.; Abdel-Karim, A.; Faki, B.; Luque-Alled, J.M.; Alberto, M.; Vijayaraghavan, A.; Holmes, S.M.; Szekely, G.; Badawy, M.I.; Shokri, N.; et al. Flux-enhanced PVDF mixed matrix membranes incorporating APTS-functionalized graphene oxide for membrane distillation. J. Memb. Sci. 2018, 554, 309–323. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Sun, W.; Wang, L.; Wu, T.; Wang, M.; Yang, Z.; Pan, Y.; Liu, G. Inhibiting the corrosion-promotion activity of graphene. Chem. Mater. 2015, 27, 2367–2373. [Google Scholar] [CrossRef]
- Diagboya, P.N.; Mmako, H.K.; Dikio, E.D.; Mtunzi, F.M. Synthesis of amine and thiol dual functionalized graphene oxide for aqueous sequestration of lead. J. Environ. Chem. Eng. 2019, 7, 103461. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ahmadi, A.; Mahdavian, M. Enhancement of the corrosion protection performance and cathodic delamination resistance of epoxy coating through treatment of steel substrate by a novel nanometric sol-gel based silane composite film filled with functionalized graphene oxide nanosheets. Corros. Sci. 2016, 109, 182–205. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, J.D.; Cruz, J.C.; Reyes, L.H. Synthesis, Characterization, and Functionalization of Graphene Oxide-Based Nanoplatforms for Gene Delivery. Mater. Proc. 2021, 4, 23. https://doi.org/10.3390/IOCN2020-07925
Torres JD, Cruz JC, Reyes LH. Synthesis, Characterization, and Functionalization of Graphene Oxide-Based Nanoplatforms for Gene Delivery. Materials Proceedings. 2021; 4(1):23. https://doi.org/10.3390/IOCN2020-07925
Chicago/Turabian StyleTorres, Julián D., Juan C. Cruz, and Luis H. Reyes. 2021. "Synthesis, Characterization, and Functionalization of Graphene Oxide-Based Nanoplatforms for Gene Delivery" Materials Proceedings 4, no. 1: 23. https://doi.org/10.3390/IOCN2020-07925
APA StyleTorres, J. D., Cruz, J. C., & Reyes, L. H. (2021). Synthesis, Characterization, and Functionalization of Graphene Oxide-Based Nanoplatforms for Gene Delivery. Materials Proceedings, 4(1), 23. https://doi.org/10.3390/IOCN2020-07925







