Bubble Behavior on Horizontal and Vertical Carbon Anode Surfaces in Cryolite Melt Applying a See-Through Cell †
Abstract
:1. Introduction
2. Experimental Part—Materials and Methods
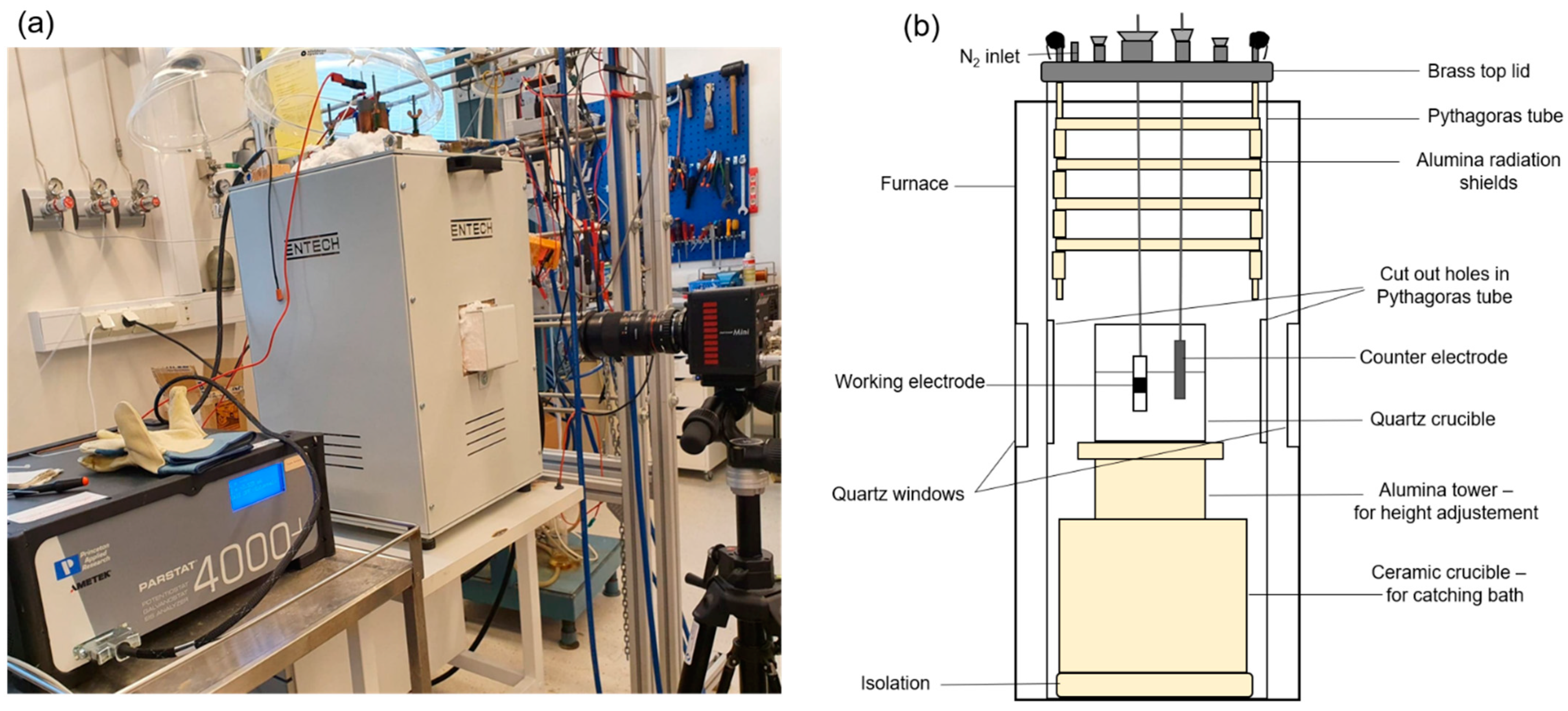
2.1. See-Through Cell
2.2. Bath Composition and Temperature Control
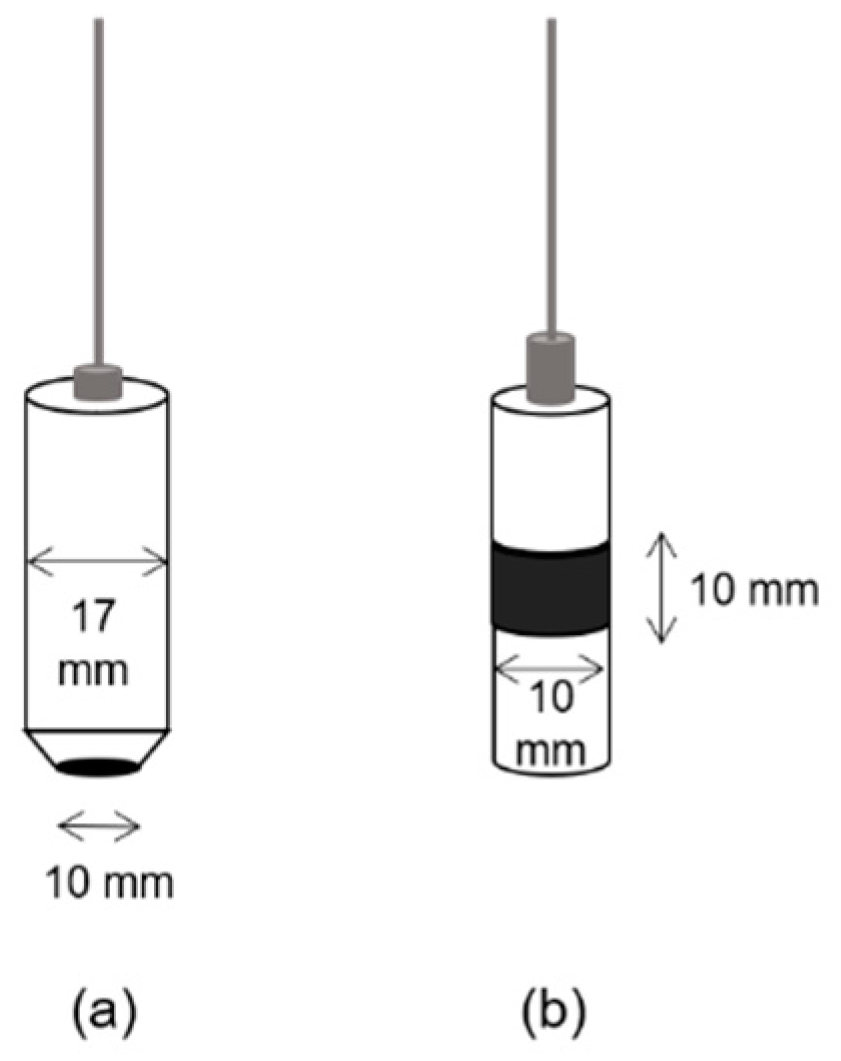
2.3. Anode Design
2.4. Experimental Methods
3. Results and Discussion
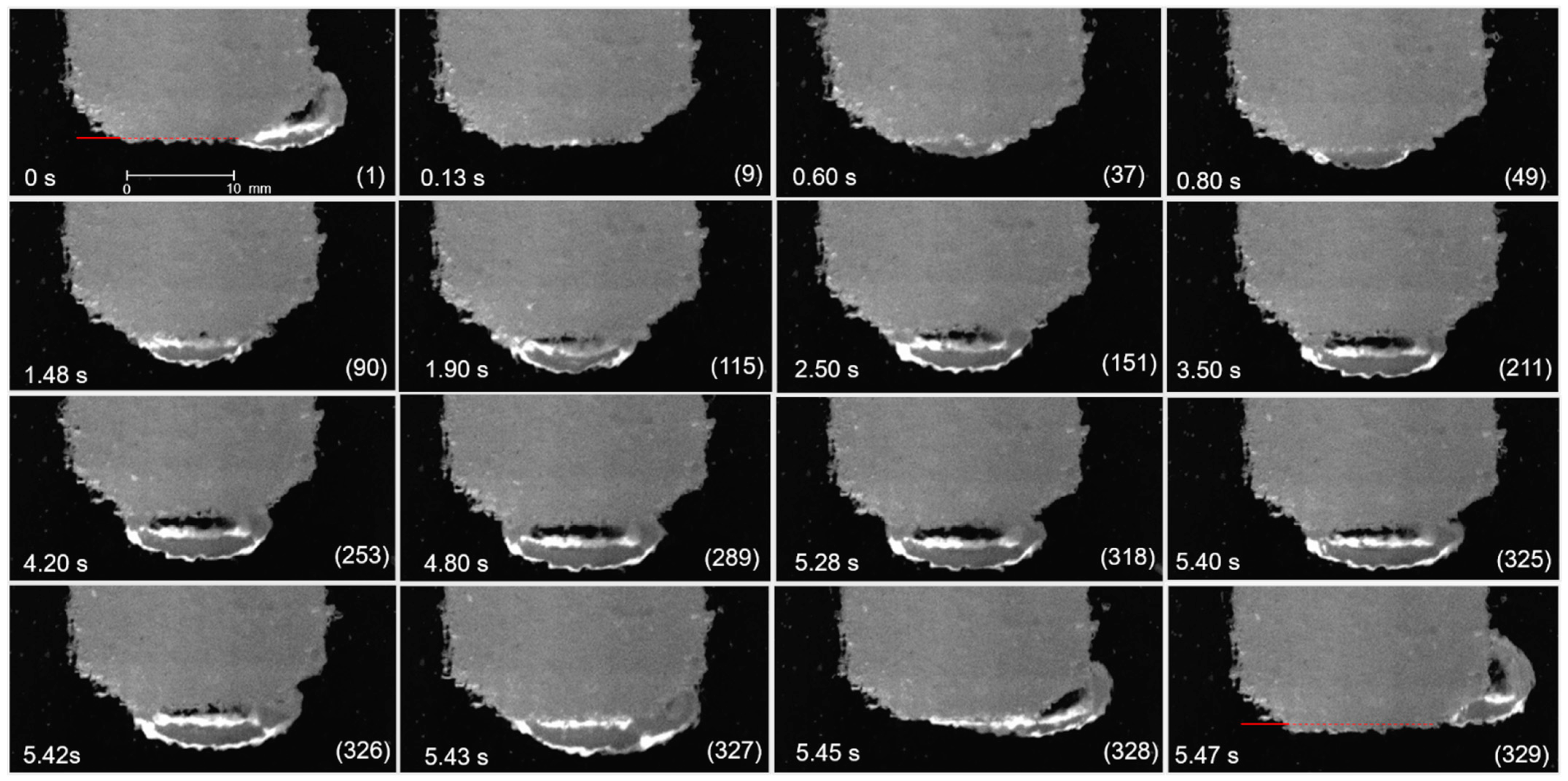
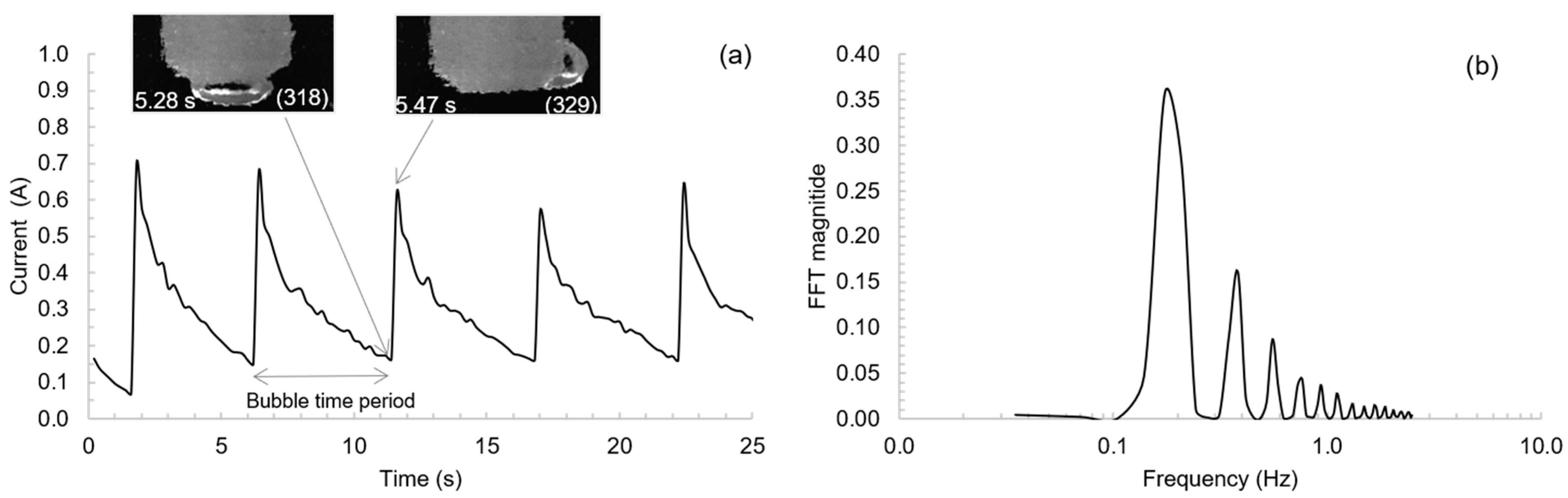
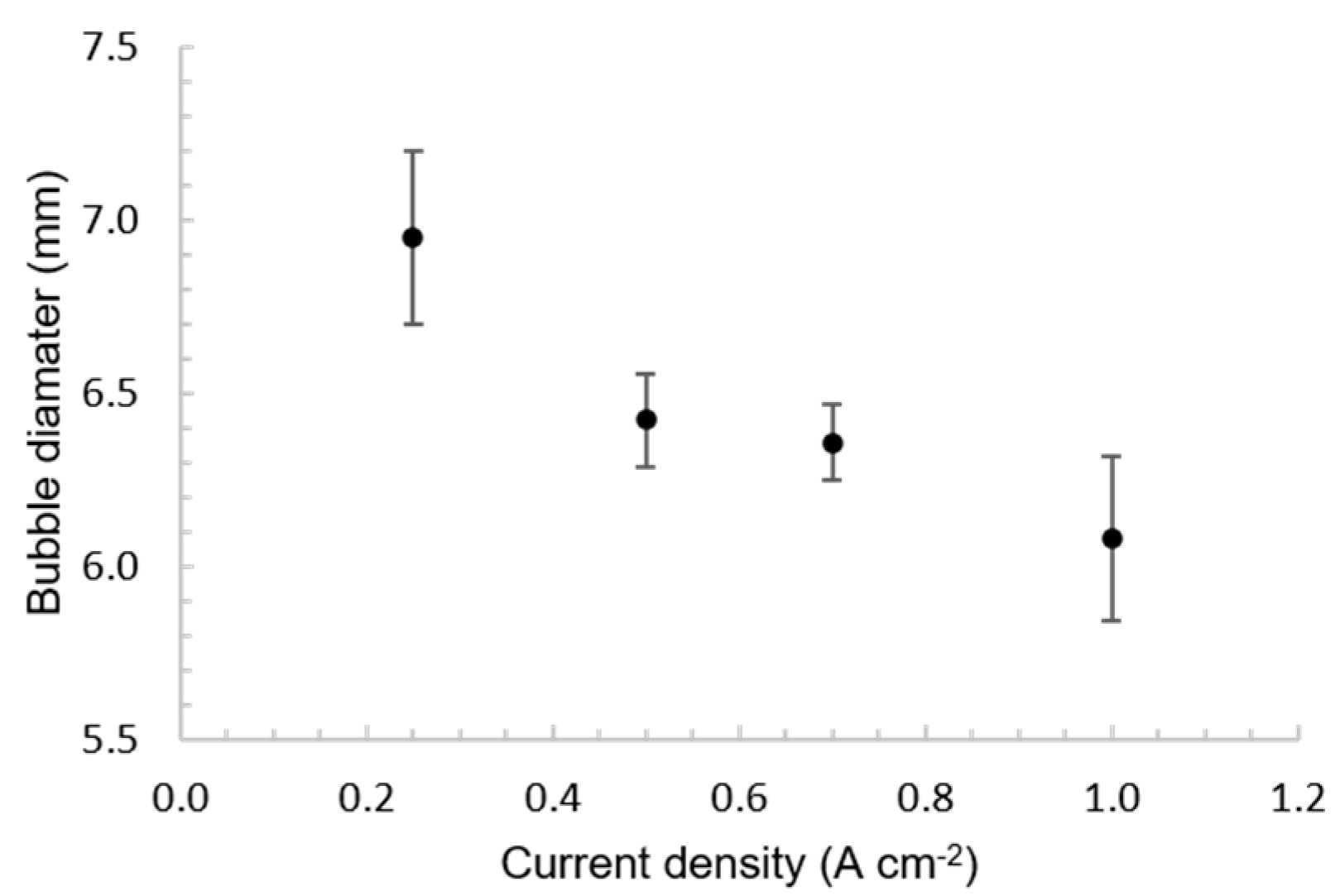
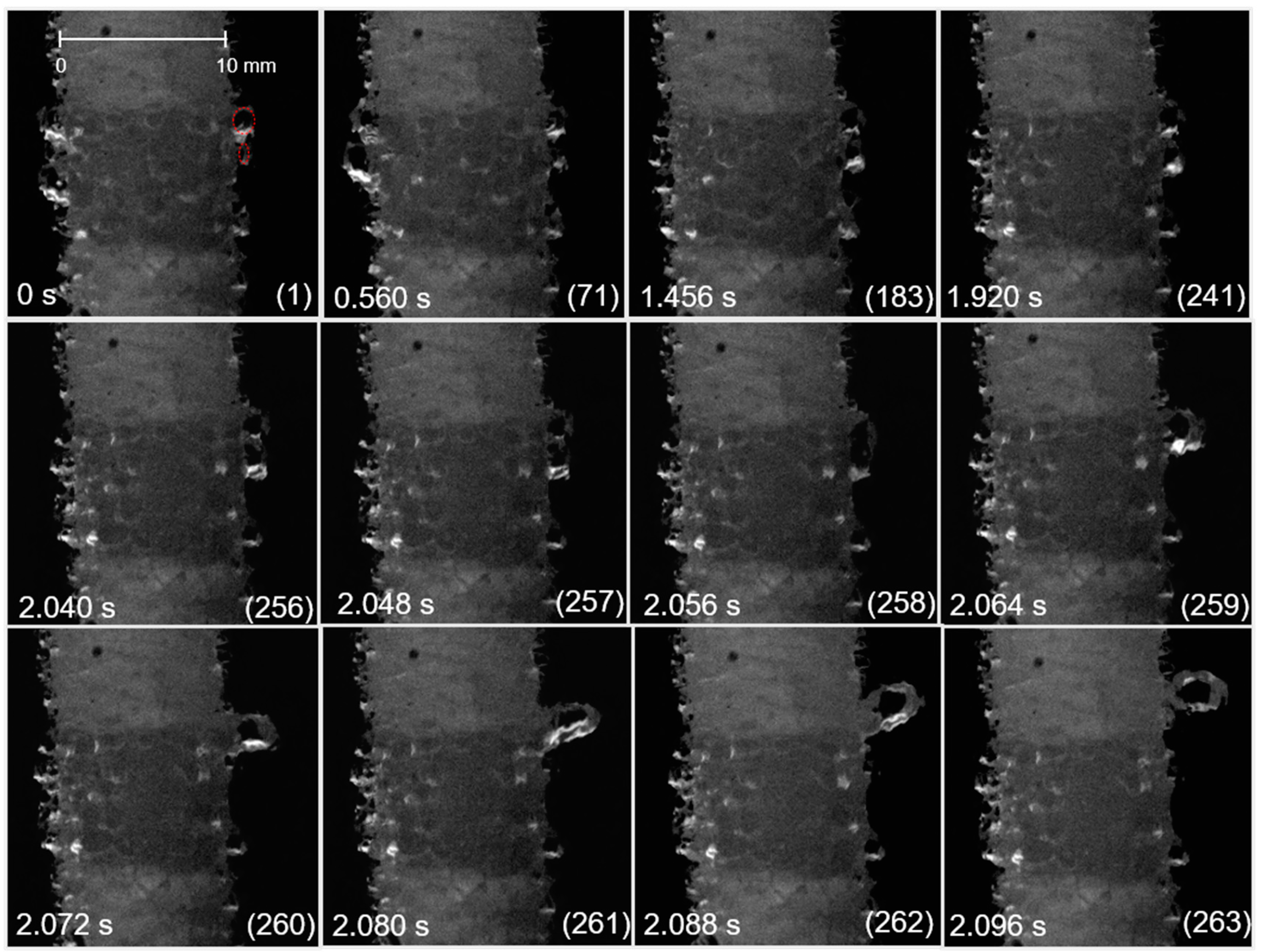
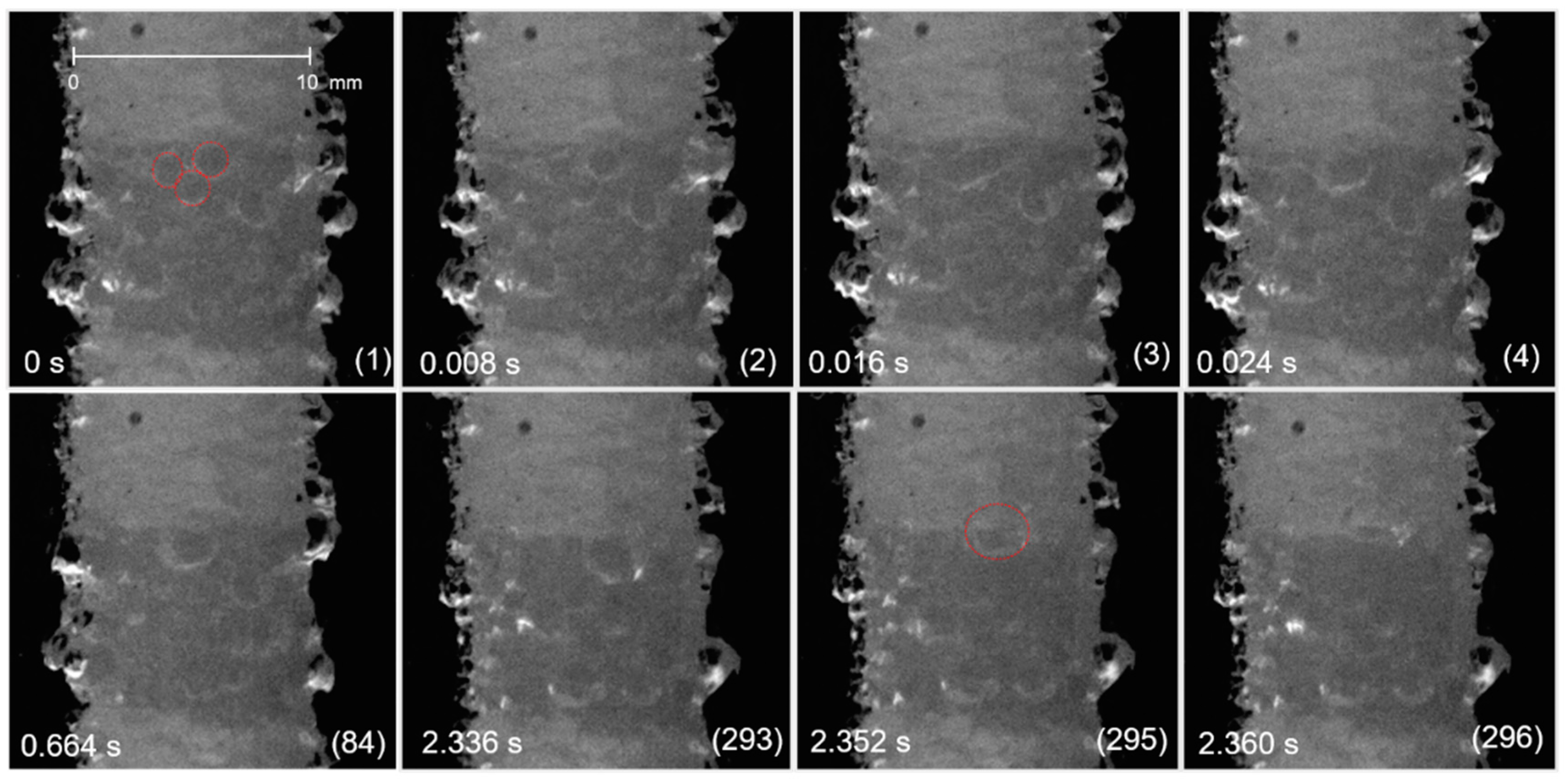
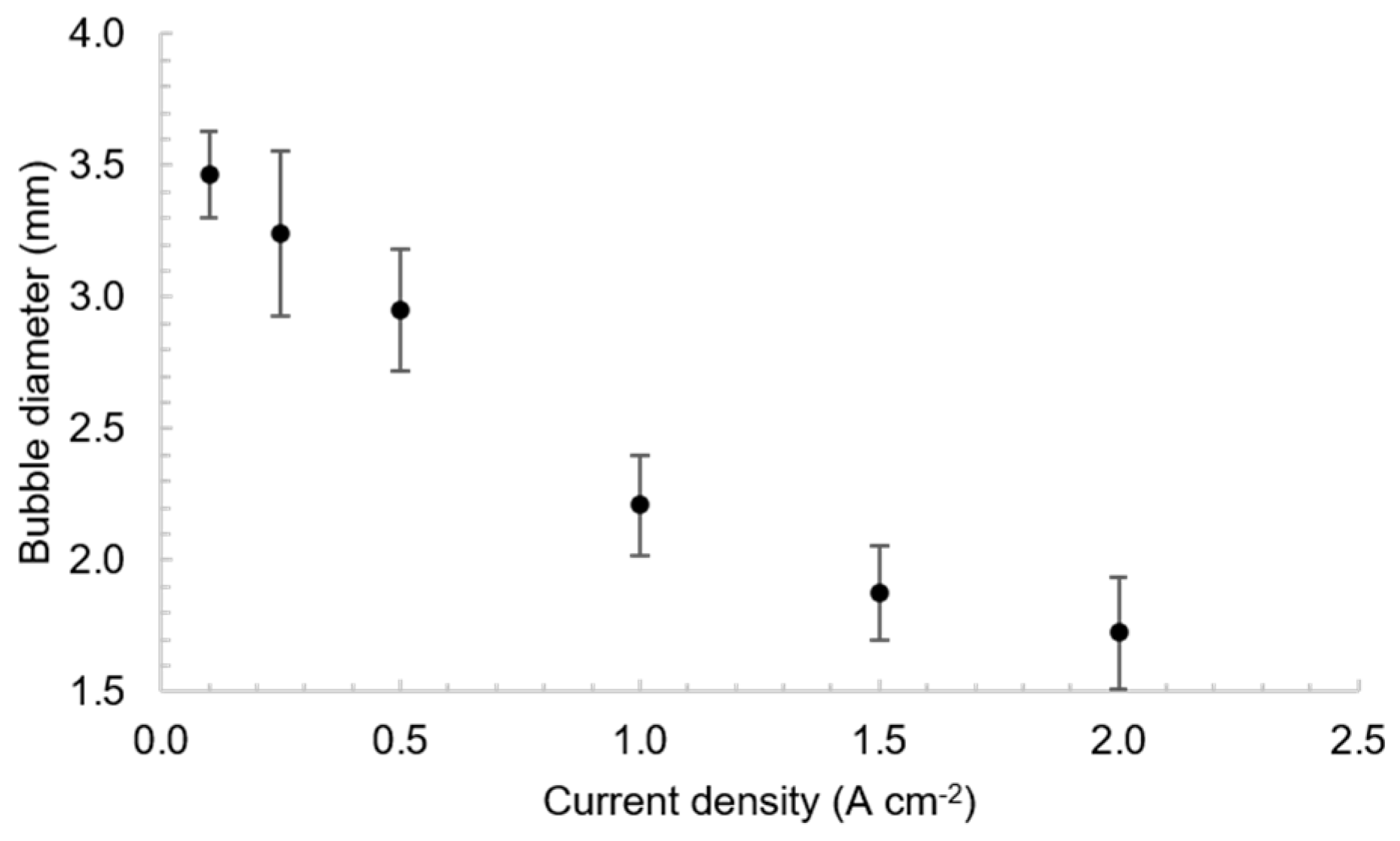
3.1. Bubble Behavior on the Horizontal Anode
3.2. Bubble Behavior on the Vertical Anode
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
0.736(mass%LiF)1.3 + 0.063((mass%AlF3) * (mass%LiF))1.1 − 3.19(mass%CaF2) + 0.03(mass%CaF2)2 +
0.27((mass%AlF3) * (mass%CaF2))0.7 − 12.2(mass%Al2O3) + 4.75(mass%Al2O3)1.2
References
- Zhao, Z.; Wang, Z.; Gao, B.; Feng, Y.; Shi, Z.; Hu, X. Observation of Anodic Bubble Behavior Using Laboratory Scale Transparent Aluminium Electrolysis Cells. In Light Metals 2015; Hyland, M., Ed.; Springer: Cham, Switzerland, 2015; pp. 801–806. [Google Scholar] [CrossRef]
- Einarsrud, K.E. A Treatise on Interpolar Transport Phenomena. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2012. [Google Scholar]
- Cassayre, L.; Utigard, T.; Bouvet, S. Visualizing gas evolution on graphite and oxygen-evolving anodes. JOM 2002, 54, 41–45. [Google Scholar] [CrossRef]
- Cassayre, L.; Plascencia, G.; Marin, T.; Fan, S.; Utigard, T. Gas Evolution on Graphite and Oxygen-Evolving Anodes During Aluminium Electrolysis. In Light Metals 2006; Galloway, T.J., Ed.; TMS: Warrendale, PA, USA, 2006; pp. 379–383. [Google Scholar]
- Xue, J.; Øye, H.A. Bubble behaviour: Cell voltage oscillation during aluminium electrolysis and the effects of sound and ultrasound. In Light Metals 1995; Evans, J., Ed.; TMS: Warrendale, PA, USA, 1995; pp. 265–271. [Google Scholar]
- Zhao, Z.; Wang, Z.; Gao, B.; Feng, Y.; Shi, Z.; Hu, X. Anodic Bubble Behavior and Voltage Drop in a Laboratory Transparent Aluminum Electrolytic Cell. Metall. Mater. Trans. B 2016, 47, 1962–1975. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Z.; Yang, Y.; Gao, B.; Shi, Z.; Hu, X. Anodic Bubble Behavior in a Laboratory Scale Transparent Electrolytic Cell for Aluminum Electrolysis. Metals 2018, 8, 806. [Google Scholar] [CrossRef]
- Stanic, N.; Jevremovic, I.; Martinez, A.M.; Sandnes, E. Bubble Evolution on Different Carbon Anode Designs in Cryolite Melt. Metall. Mater. Trans. B 2020, 51, 1243–1253. [Google Scholar] [CrossRef]
- Leistra, J.; Sides, P.; Leistra, J. Hyperpolarization at Gas Evolving Electrodes. II. Hall/Heroult Electrolysis. Electrochim. Acta 1988, 33, 1761–1766. [Google Scholar] [CrossRef]
- Cooksey, M.; Taylor, M.; Chen, J. Resistance due to gas bubbles in aluminum reduction cells. JOM 2008, 60, 51–57. [Google Scholar] [CrossRef]
- Åsheim, H.; Eidsvaag, I.A.; Solheim, A.; Gudbrandsen, H.; Haarberg, G.M.; Sandnes, E. The Influence of Polarisation on the Wetting of Graphite in Cryolite-Alumina Melts. In Light Metals 2020; Springer: Cham, Switzerland, 2010; pp. 608–619. [Google Scholar]
- Grjotheim, K.; Kvande, H. Introduction to Aluminium Electrolysis: Understanding the Hall-Héroult Process, 2nd ed.; Aluminium-Verlag: Düsseldorf, Germany, 1993. [Google Scholar]
- Haupin, W. The Liquidus Enigma. In Light Metals 1992: Proceedings of the Technical Sessions Presented by the TMS Light Metals Committee at the 121st TMS Annual Meeting, San Diego, California, 1–5 March 1992; Cutshall, E.R., Ed.; Minerals, Metals & Materials Society: Warrendale, PA, USA, 1991; pp. 477–480. [Google Scholar]
| wt% | Specification | Producer | |
|---|---|---|---|
| Al2O3 | 3 | γ-alumina | Merck |
| AlF3 | 15 | sublimed “in house” | Industrial grade |
| LiF | 15 | purum | Riedel-de-Haën |
| CaF2 | 5 | precipitated pure | Merck |
| Cryolite | 62 | synthetic, purity > 97% | Sigma-Aldrich |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanic, N.; Sandnes, E. Bubble Behavior on Horizontal and Vertical Carbon Anode Surfaces in Cryolite Melt Applying a See-Through Cell. Mater. Proc. 2021, 3, 8. https://doi.org/10.3390/IEC2M-09238
Stanic N, Sandnes E. Bubble Behavior on Horizontal and Vertical Carbon Anode Surfaces in Cryolite Melt Applying a See-Through Cell. Materials Proceedings. 2021; 3(1):8. https://doi.org/10.3390/IEC2M-09238
Chicago/Turabian StyleStanic, Nikolina, and Espen Sandnes. 2021. "Bubble Behavior on Horizontal and Vertical Carbon Anode Surfaces in Cryolite Melt Applying a See-Through Cell" Materials Proceedings 3, no. 1: 8. https://doi.org/10.3390/IEC2M-09238
APA StyleStanic, N., & Sandnes, E. (2021). Bubble Behavior on Horizontal and Vertical Carbon Anode Surfaces in Cryolite Melt Applying a See-Through Cell. Materials Proceedings, 3(1), 8. https://doi.org/10.3390/IEC2M-09238







