Silver Nanostars Spread on Cu(OH)2 Nanowires for SERS Substrates †
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Instruments
2.3. Silver Nanostar Synthesis
2.4. Preparation of SERS Substrates
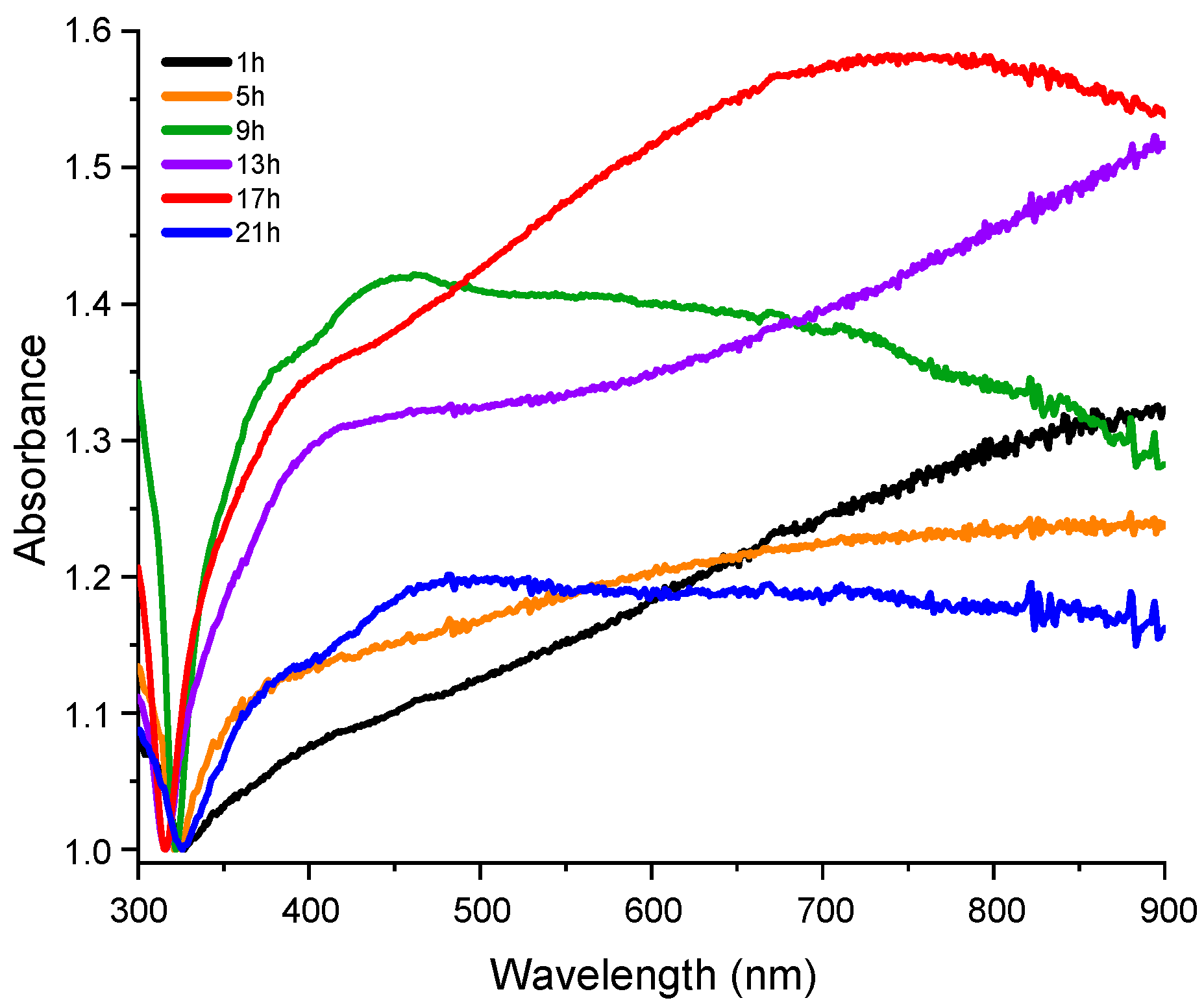
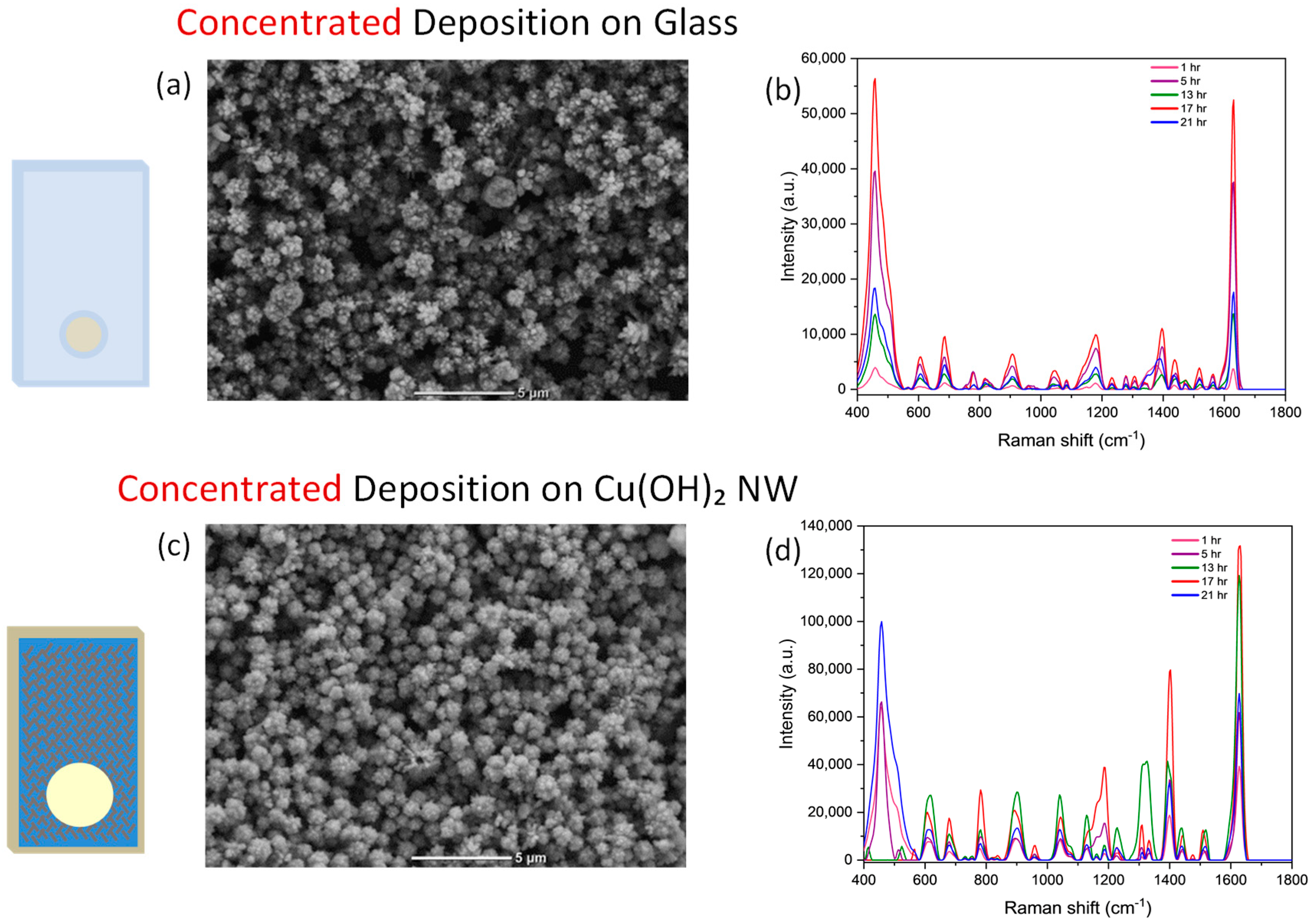
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le Ru, E.C.; Etchegoin, P.G. Principles of Surface-Enhanced Raman Spectroscopy: And Related Plasmonic Effects; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Garcia-Leis, A.; García-Ramos, J.V.; Sanchez-Cortes, S. Silver Nanostars with High SERS Perfomance. J. Front. Chem. 2013, 117, 7791–7795. [Google Scholar] [CrossRef]
- Cañamares, M.V.; García-Ramos, J.V.; Sanchez-Cortes, S.; Castillejo, M.; Oujja, M. Comparative SERS effectiveness of silver nanoparticles prepared by different methods: A study of the enhancement factor and the interfacial properties. J. Colloid Interface Sci. 2008, 326, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Khoury, C.G.; Vo-Dinh, T. Gold nanostars for surface-enhanced Raman scattering: Synthesis, characterization and optimization. J. Phys. Chem. C 2008, 112, 18849–18859. [Google Scholar] [CrossRef] [PubMed]
- Bakar, N.A.; Shapter, J.G. Silver nanostars films for surface-enhanced Raman spectroscopy (SERS) of the pesticide imidacloprid. Heliyon 2023, 9, e14686. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, Q.; Yin, N.; Xue, Z.; Sun, Y.; Wu, Y. Self-assembled bimetallic Au@Ag nanostars monolayer flexible substrates for ultrasensitive SERS. Anal. Chim. Acta 2025, 1043, 184277. [Google Scholar] [CrossRef]
- Zhou, Q.; Meng, G.; Liu, J.; Huang, Z.; Han, F.; Zhu, C.; Kim, D.; Kim, T.; Wu, N. A Hierarchical Nanostructure-Based Surface-Enhanced Raman Scattering Sensor for Preconcentration and Detection of Antibiotic Pollutants. Adv. Mat. Technol. 2017, 2, 1700028. [Google Scholar] [CrossRef]
- Guo, T.L.; Li, J.G.; Sun, X.; Sakka, Y. Improved galvanic replacement growth of Ag microstructures on Cu micro-grid for enhanced SERS detection of organic molecules. Mater. Sci. Eng. 2016, 61, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, N.N.; Nor Azmi, F.S.; Abu Bakar, N.; Aziz, T.H.T.A.; Shapter, J.G. Titanium carbide MXene/silver nanostars composite as SERS substrate for thiram pesticide detection. Chem. Pap. 2024, 78, 2855–2865. [Google Scholar] [CrossRef]
- De Almeida, M.P.; Rodrigues, C.; Novais, A.; Grosso, F.; Leopold, N.; Peixe, L.; Franco, R.; Pereira, E. Silver Nanostar-Based SERS for the Discrimination of Clinically Relevant Acinetobacter baumannii and Klebsiella pneumoniae Species and Clones. Biosensors 2023, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Chen, T.; Qian, Y.; Huang, Q.; Wei, T.; Hu, X.; Zhao, J.; Wang, B. A gold–silver nanostar 2D array composite structure for rapid SERS determination of PAHs. Anal. Methods 2025, 18, 3825–3835. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Navarro, J.L.; Gonzalez-Zarate, D.; Diaz-Solis, M.A.; Soriano-Rosales, M.G.; Okolodkov, Y.B.; Zamora-Peredo, L. SERS Detection of Methylene Blue and Crystal Violet Using Silver Nanostars. Mater. Proc. 2022, 9, 27. [Google Scholar]
- Diaz Solis, M.A.; Pinilla Rodriguez, J.A.; Pozos Texon, F.d.J.; Delfín Ruiz, M.E.; Zamora Peredo, L.; Hernández Torres, J. Assessment via SERS of Two Simple Chemical Methods for Silver Nanoparticle Deposition Onto Copper Hydroxide Nanowires. In Proceedings of the 2023 IEEE International Conference on Engineering Veracruz (ICEV), Boca del Río, Veracruz, Mexico, 23–26 October 2023; pp. 1–5. [Google Scholar]
- Xu, T.; Wang, X.; Huang, Y.; Lai, K.; Fan, Y. Rapid detection of trace methylene blue and malachite green in four fish tissues by ultra-sensitive surface-enhanced Raman spectroscopy coated with gold nanorods. Food Control 2019, 106, 106720. [Google Scholar] [CrossRef]
- Tuschel, D. Selecting an excitation wavelength for Raman spectroscopy. Spectroscopy 2016, 3, 14–23. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, J.L.Z.; Girón, D.J.; Rodríguez, H.A.R.; Okolodkov, Y.; Cervantes, M.L.; Rodríguez, G.S.; Torres, J.H.; Peredo, L.Z. Silver Nanostars Spread on Cu(OH)2 Nanowires for SERS Substrates. Mater. Proc. 2025, 28, 7. https://doi.org/10.3390/materproc2025028007
Navarro JLZ, Girón DJ, Rodríguez HAR, Okolodkov Y, Cervantes ML, Rodríguez GS, Torres JH, Peredo LZ. Silver Nanostars Spread on Cu(OH)2 Nanowires for SERS Substrates. Materials Proceedings. 2025; 28(1):7. https://doi.org/10.3390/materproc2025028007
Chicago/Turabian StyleNavarro, José Luis Zamora, Diana Jiménez Girón, Hector Ariel Renteral Rodríguez, Yuri Okolodkov, Marcos Luna Cervantes, Guillermo Santana Rodríguez, Julián Hernández Torres, and Luis Zamora Peredo. 2025. "Silver Nanostars Spread on Cu(OH)2 Nanowires for SERS Substrates" Materials Proceedings 28, no. 1: 7. https://doi.org/10.3390/materproc2025028007
APA StyleNavarro, J. L. Z., Girón, D. J., Rodríguez, H. A. R., Okolodkov, Y., Cervantes, M. L., Rodríguez, G. S., Torres, J. H., & Peredo, L. Z. (2025). Silver Nanostars Spread on Cu(OH)2 Nanowires for SERS Substrates. Materials Proceedings, 28(1), 7. https://doi.org/10.3390/materproc2025028007




