Optimizing a Cu-Ni Nanoalloy-Coated Mesoporous Carbon for Efficient CO2 Electroreduction † †
Abstract
1. Introduction
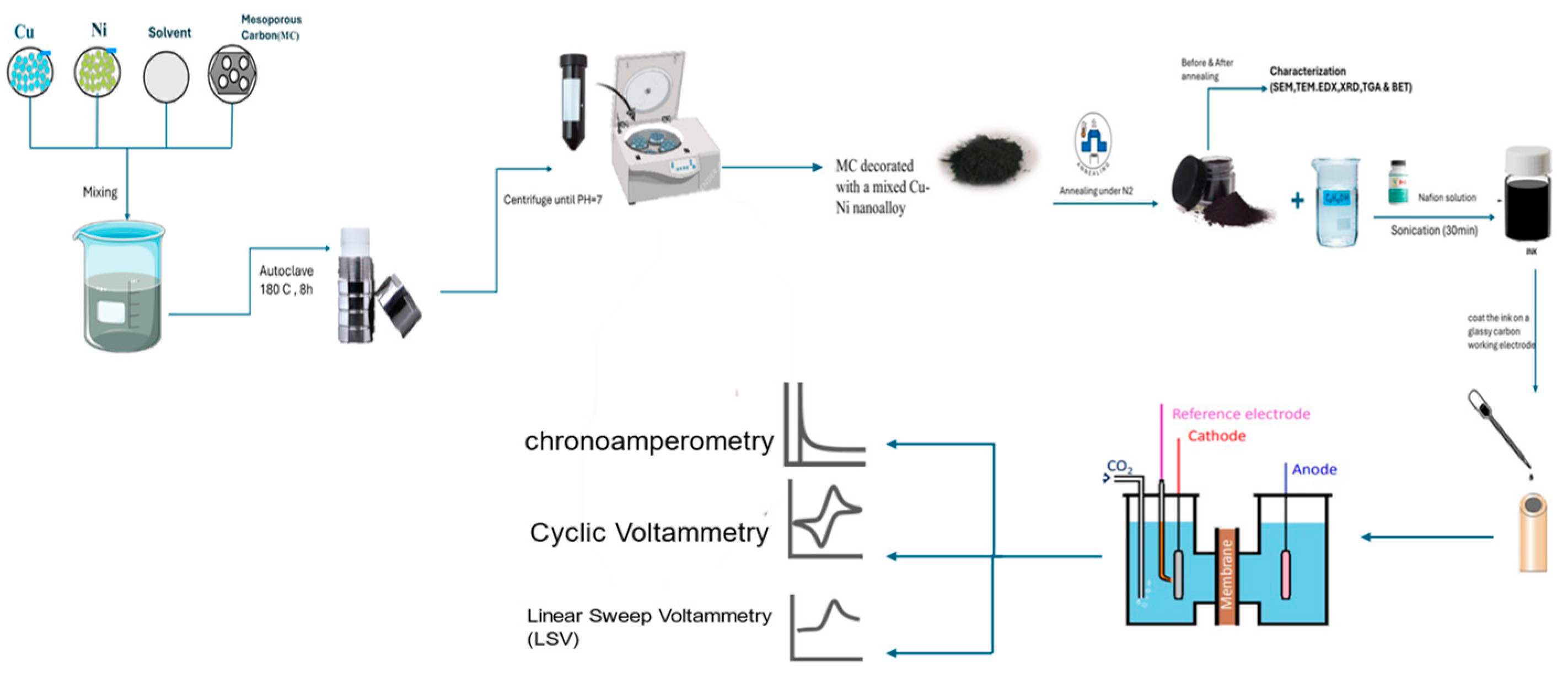
2. Materials and Methods
3. Results and Discussion
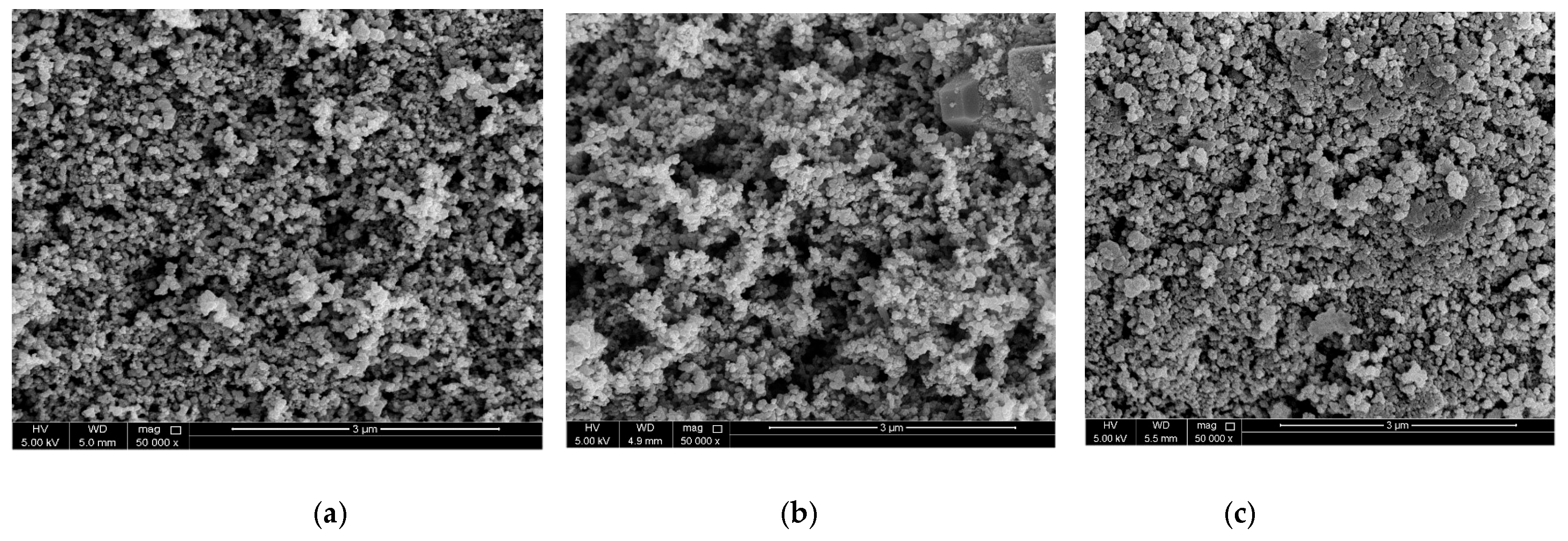
3.1. Morphology and Structure Analysis
3.2. Phase and Composition Analysis
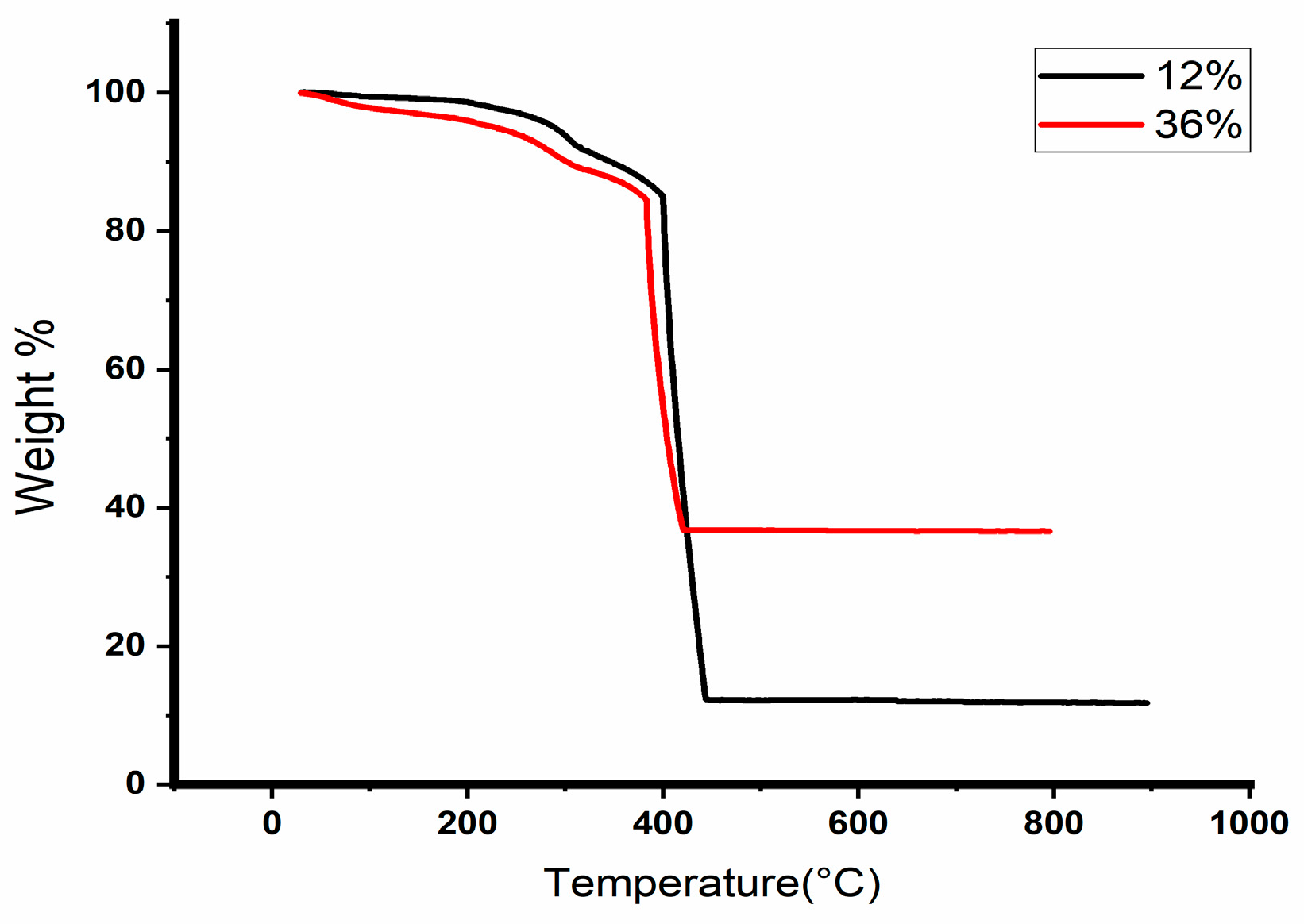
3.3. Thermal Stability
3.4. Electrochemical CO2 Reduction Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tirumala, R.T.A.; Ramakrishnan, S.B.; Andiappan, M. Distinguishing Dynamic Phase Catalysis in Cu based nanostructures under Reverse Water Gas Shift Reaction. arXiv 2022. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, T.; Dong, L.; Qin, Z.; Ji, H. Ni/bentonite catalysts prepared by solution combustion method for CO2 methanation. Chin. J. Chem. Eng. 2018, 26, 2361–2367. [Google Scholar] [CrossRef]
- Wei, X.; Li, Z.; Jang, H.; Kim, M.G.; Qin, Q.; Liu, X. Lattice strain and interfacial engineering of a Bi-based electrocatalyst for highly selective CO2 electroreduction to formate. Sci. China Mater. 2023, 66, 1398–1406. [Google Scholar] [CrossRef]
- Han, W.; Liu, B.; Chen, Y.; Jia, Z.; Wei, X.; Song, W. Coordinatively unsaturated aluminum anchored Ru cluster for catalytic hydrogenation of benzene. J. Catal. 2021, 400, 255–264. [Google Scholar] [CrossRef]
- Tan, D.; Zhang, J.; Cheng, X.; Tan, X.; Shi, J.; Zhang, B.; Han, B.; Zheng, L.; Zhang, J. CuxNiy alloy nanoparticles embedded in a nitrogen–carbon network for efficient conversion of carbon dioxide. Chem. Sci. 2019, 10, 4491–4496. [Google Scholar] [CrossRef] [PubMed]
- Bagger, A.; Ju, W.; Varela, A.S.; Strasser, P.; Rossmeisl, J. Electrochemical CO2 Reduction: Classifying Cu Facets. ACS Catal. 2019, 9, 7894–7899. [Google Scholar] [CrossRef]
- Meng, X.; Pan, G.; Liu, H.; Qian, Y.; Wang, X.; Wang, C.; Hu, L.; Wang, H.; Chen, Q. Ultrasmall Cu Nanocrystals Dispersed in Nitrogen-Doped Carbon as Highly Efficient Catalysts for CO2 Electroreduction. ACS Appl. Mater. Interfaces 2022, 14, 17240–17248. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, S.; Pastor, E.; Lázaro, M.J. Ordered Mesoporous Carbon as a Support of Pd Catalysts for CO2 Electrochemical Reduction. Catalysts 2020, 10, 912. [Google Scholar] [CrossRef]
- He, C.; Wang, S.; Jiang, X.; Hu, Q.; Yang, H.; He, C. Bimetallic Cobalt–Copper Nanoparticle-Decorated Hollow Carbon Nanofibers for Efficient CO2 Electroreduction. Front. Chem. 2022, 10, 904241. [Google Scholar] [CrossRef] [PubMed]


| Reference | Nanoalloy Composition | Nanoalloy Size/Facets | Metal–Support Interaction | Mesoporous Carbon Properties |
|---|---|---|---|---|
| [1] | Cu: XNiy alloys | Nanoparticles | Nitrogen–carbon network | - |
| [2] | Cu | Different facets | - | - |
| [3] | Cu | Ultrasmall nanocrystals | Nitrogen-doped carbon | - |
| [4] | Pd | - | Ordered mesoporous carbon | High surface area, ordered pores |
| [5] | CuNi | Nanoparticles | Hollow carbon nanofibers | Conductive support |
| This Work | CuNi | Bimetal | Coated mesoporous Carbon | High surface area Small particle size |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhamdan, M.B.; Radwan, A.B.; Al-Qahtani, N.
Optimizing a Cu-Ni Nanoalloy-Coated Mesoporous Carbon for Efficient CO2 Electroreduction
Alhamdan MB, Radwan AB, Al-Qahtani N.
Optimizing a Cu-Ni Nanoalloy-Coated Mesoporous Carbon for Efficient CO2 Electroreduction
Alhamdan, Manal B., Ahmed Bahgat Radwan, and Noora Al-Qahtani.
2025. "Optimizing a Cu-Ni Nanoalloy-Coated Mesoporous Carbon for Efficient CO2 Electroreduction
Alhamdan, M. B., Radwan, A. B., & Al-Qahtani, N.
(2025). Optimizing a Cu-Ni Nanoalloy-Coated Mesoporous Carbon for Efficient CO2 Electroreduction





