1. Introduction
Recently, rare earth metals (REEs) have come to be regarded as strategic raw materials for high-tech industry in the 21st century. Despite the many advantages of their properties, rare earth metals still have certain limitations: Toxicity: Some rare earth metals are toxic to humans and the environment. For example, gadolinium can cause kidney problems, dysprosium can affect the heart, and lanthanum can cause skin and eye irritation. The extraction and processing of rare earth metals is also associated with the risk of environmental pollution. The complexity of recycling: Rare earth metals are very difficult to recycle, which leads to them often being released into the environment after use. The recycling process is also expensive and energy intensive.
Therefore, the use of a non-toxic carrier such as SiO
2 is a solution that should be considered. In 1956, Gerhard Kolbe opened a new era in the synthesis of spherical particles of silicon dioxide by hydrolyzing tetraethoxysilane (TEOS) in an alcoholic solution and using ammonia as an alkaline catalyst [
1]. The modified Stober method involves the hydrolysis of tetraethoxysilane (TEOS) in an alcoholic solution in the presence of ammonia (catalyst). An improved version of the Stober method was proposed by Stober, Fink and Bohn in 1968. It allows the obtainment of particles with sizes from 50 nm to 2000 nm with high monodispersity [
2]. The advantage is the simplicity of the SiO
2 synthesis process and the production of particles with a narrow size distribution (from 100 nm to 3000 nm), but there are still limitations, such as the difficulty of obtaining high monodispersity for particles less than 100 nm. The heterogeneous hydrolysis of TEOS with amino acids was performed via the hydrolysis of TEOS under slightly alkaline conditions (pH 9–10) in an emulsion containing TEOS, water, and an amino acid (L-lysine). This was developed by Yokoi T. and co-authors (2006) and Davis T.M. with co-authors (2006). It allows the obtainment of spherical silica nanoparticles with a diameter of 10–20 nm with high monodispersity [
3,
4]. A combination of the advantages of Stober’s methods with heterogeneous hydrolysis with amino acids was developed by Hartlen K.D. and co-authors (2008). The heterogeneous hydrolysis of TEOS with L-arginine was used to obtain a seed, and seed growth was promoted by the Stober method. This allowed the obtainment of particles in a wide range of sizes (from 15 nm to several microns) with a high degree of uniformity (<5%) [
5].
To understand the nature and mechanism of processes occurring on the surface of a solid body, it is necessary to have a set of experimental data characterizing the surface properties, such as the chemical composition of surface compounds, phase state, and physical and chemical characteristics [
6]. Acid-base properties are one of the characteristics that best reflect the reactivity of the surface in donor–acceptor interactions [
7]. The chemical and energetic heterogeneity of the surface of solids is determined by the presence of structural defects and functional groups of different compositions on the surface, which manifest themselves as active centers in adsorption and chemical reactions [
8]. In this connection, the study of the composition and content of active centers makes it possible to predict the reactivity and sorption capacity of the surface. So, in this study, we focused our research on determining the kinetics of the synthesis of the SiO
2-Gd
2O
3-Eu
2O
3 system under tetraethoxysilane hydrolysis conditions in water and researching acid-base properties of samples obtained from TEOS, europium, and gadolinium nitrate solutions using different solvents: citric acid and ethanol.
2. Materials and Methods
To research the kinetics of the formation of system SiO2-Gd2O3-Eu2O3 in water it was necessary to prepare 0.1 M Gd(NO3)3, 0.1 M Eu(NO3)3, and tetraethoxysilane (TEOS) with different SiO2-Gd2O3-Eu2O3 composition ratios: 100:0:0; 95:3:2; 92:5:3; 90:6:4. These mixtures with these different ratios were added to 100 mL of water, and the solution was heated to a predetermined temperature in a thermostat with a magnetic stirrer. The solution temperatures of 35, 45, and 55 °C were kept constant with an accuracy of ±1 °C throughout the synthesis time. The rotational speed of the magnetic stirrer was kept the same in all experiments. During the synthesis, the colloidal system was sampled to determine the mass concentration. The mass concentration of the substance in the aqueous part of the reaction mixture was determined by the ratio of the mass of particles that were dried and annealed at 700 °C that were contained in the sample to the mass of the suspension sample.
To study the acid-base properties of the powder surface of SiO2-Gd2O3-Eu2O3, we used the Hammett indicator adsorption method. The adsorption of indicators on the surface of the system allows us to determine the qualitative composition and concentration of active centers on the oxide surface, as well as the distribution of active centers by strength. Three samples were obtained from the solutions of TEOS, europium, and gadolinium nitrate using different solvents: citric acid and ethanol.
Sample 1: Method with citric acid after Lyophilic drying.
Sample 2: Method with ethanol after Lyophilic drying.
Sample 3: Method with ethanol after natural air drying.
The solutions prepared in this way were used for further studies on a spectrophotometer, UNICO 2100. The set of used indicators allowed us to register acid-base properties in the range of 2.1 to 9.4 pKa at a maximum indicator absorption wavelength in 3 mm-thick cells.
3. Results
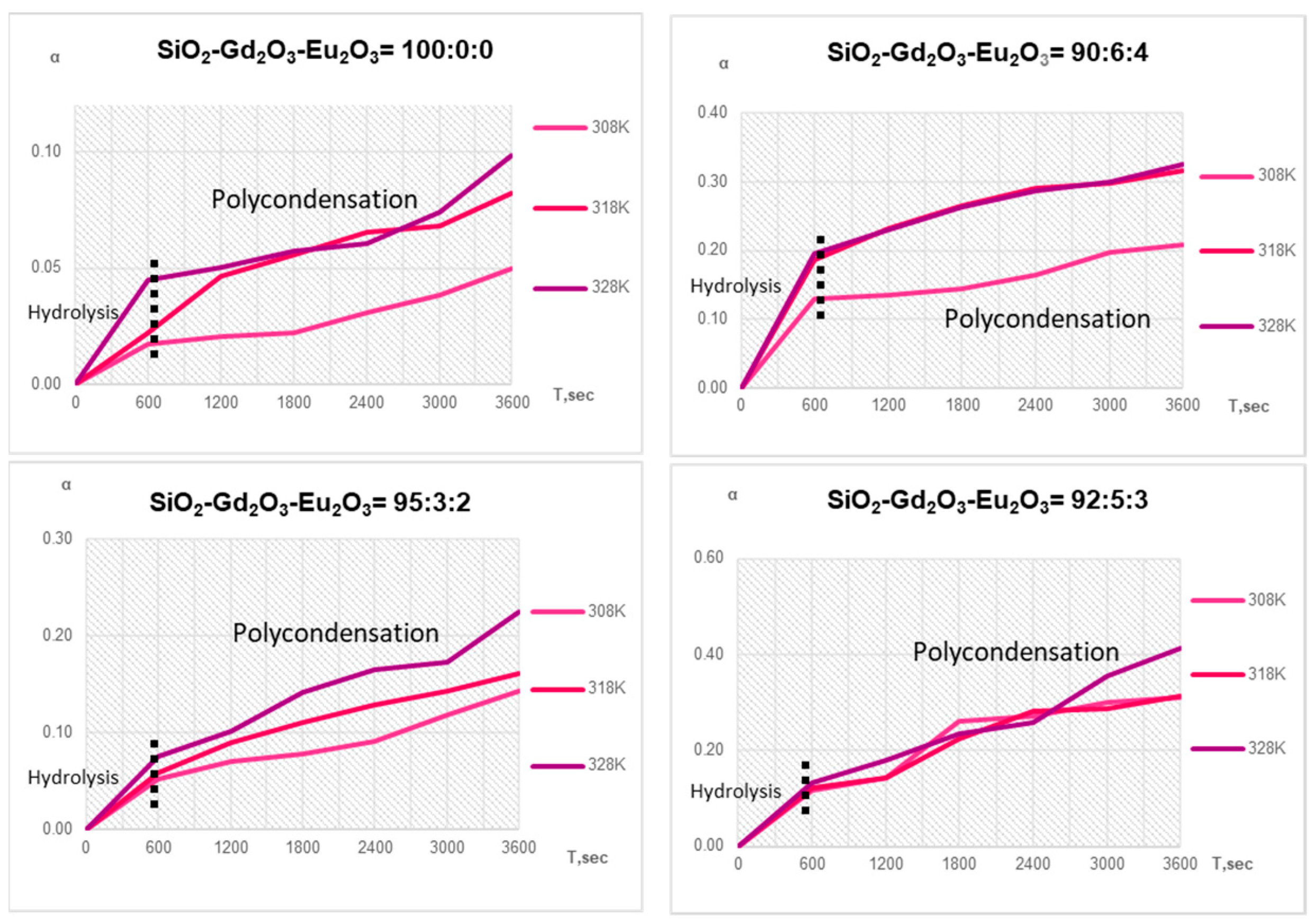
Figure 1: The degree of reaction of the stage of hydrolysis increases from 2 to 20% with an increase in the concentration of Gd
2O
3-Eu
2O
3. The degree of reaction in the stage of polycondensation also increases with an increase in the content of Gd
2O
3-Eu
2O
3, but does not reach 100%; this is due to the fact that during the transformation, hydrated gadolinium and europium ions are located on the surface of silicon oxide particles and shield them from further precipitation.
Figure 2: In the process of heterogeneous hydrolysis, a layered structure was obtained: in the center is the Si(OH)
4 nucleus, then a polymer layer of Si-O-Si, and then a layer of hydrated metal ions; firing this final layer turns it into the oxides of Gd
2O
3 and Eu
2O
3.
Table 1: The activation energy of the hydrolysis process depends on the concentration of Gd
2O
3-Eu
2O
3 oxides and varies in the range of 17.65–6.03 kJ/mol. The maximum value of the activation energy is 17.65 kJ/mol for the system with the absence of rare earth elements (SiO
2-Gd
2O
3-Eu
2O
3 = 100:0:0). The minimum activation energy of the hydrolysis reaction is 6 kJ/mol for the SiO
2-Gd
2O
3-Eu
2O
3 = 92:5:3 = 92:5:3. The addition of Gd
3+ and Eu
3+ ions reduces the activation energy of silicon oxide formation from tetraethoxysilane. The europium and gadolinium ions are the catalysts for the hydrolysis stage of tetraethoxysilane.
Table 2 shows that the specific adsorption gradually increases with the pKa value of the indicator solution, increasing particularly sharply at pKa = 9.4. This proves that there are active basic centers. The concentration of major activity centers differs markedly when using different drying and synthetic methods. The best results are obtained using the Lyophilic drying method and synthetic system SiO
2-Gd
2O
3-Eu
2O
3 = 95:3:2.
4. Conclusions
The activation energy of the hydrolysis process depends on the concentration of Gd2O3-Eu2O3 oxides and varies in the range of 17.65–6.03 kJ/mol. The maximum value of the activation energy is 17.65 kJ/mol for the system with the absence of rare earth elements (SiO2:Gd2O3:Eu2O3 = 100:0:0). The addition of Gd3+ and Eu3+ ions reduces the activation energy of silicon oxide formation from tetraethoxysilane. So, these ions play the role of catalysts. The response degree of stage of hydrolysis increases from 2% to 20% with an increasing concentration of Gd2O3-Eu2O3. Therefore, the rate and completeness of hydrolysis increases with the increase in rare earth elements oxide content. The degree of the response stage of polycondensation also increases with the increasing content of Gd2O3-Eu2O3, but does not reach 100%; this is due to the fact that in the process of transformation, hydrated gadolinium and europium ions are located on the surface of silicon oxide particles, and shield these particles from further deposition. That is, in the process of heterogeneous hydrolysis, a layered structure was obtained: in the center is a Si(OH)4 nucleus, then a polymer layer of Si-O-Si, and then a layer of hydrated metal ions, which after firing turns into the oxides Gd2O3 and Eu2O3. The concentration of major activity centers differs markedly when using different drying and synthetic methods. The best results are obtained using the Lyophilic drying method and a synthetic system.
Author Contributions
S.I.N.: Explanation of results, supervision, reviewing and editing; I.V.K.: Conceptualization, methodology, explanation of results, writing of manuscript; N.A.T.: Conceptualization, methodology, explanation of results, reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data analyzed or created in the preparation of the article is included in the published article.
Conflicts of Interest
The authors confirm no conflict of interest regarding the publication of this paper.
References
- Kolbe, G. Das Complex Hemischeverhalten der Kieselsaure. Ph.D. Thesis, Friedrich-Schiller-Universität Jena, Jena, Germany, 1956. [Google Scholar]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Yokoi, T.; Sakamoto, Y.; Terasaki, O.; Kubota, Y.; Okubo, T.; Tatsumi, T. Periodic Arrangement of Silica Nanospheres Assisted by Amino Acids. J. Am. Chem. Soc. 2006, 128, 13664–13665. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.M.; Snyder, M.A.; Krohn, J.E.; Tsapatsis, M. Nanoparticles in LysineSilica Sols. Chem. Mater. 2006, 18, 5814–5816. [Google Scholar] [CrossRef]
- Hartlen, K.D.; Athanasopoulos, A.P.T.; Kitaev, V. Facile Preparation of Highly Monodisperse Small Silica Spheres (15 to >200 nm) Suitable for Colloidal Templating and Formation of Ordered Arrays. Langmuir 2008, 24, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Niftaliev, S.I.; Kuznetsova, I.V.; Saranov, I.A.; Zhundrikova, T.V.; Lygina, L.V.; Tuneekov, V.Y.; Chislova, I.V.; Zvereva, I.A. Synthesis of Nanosized Gadolinium Oxide. Glass Phys. Chem. 2019, 45, 232–237. [Google Scholar] [CrossRef]
- Tanabe, K. Solid Acids and Bases; Amazon: Moscow, Russia, 1973; pp. 67–71. [Google Scholar]
- Niftaliev, S.I.; Kuznetsova, I.V.; Lygina, L.V.; Tuneekov, V.Y.; Saranov, I.A.; Tolkacheva, A.A.; Diallo, A.; Tuken, T. Synthesis and study of nanosized gadolinium oxide modified by zirconium oxide. Solid State Sciences 2020, 110, 106457. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).