Intelligent Smart Coatings for Enhanced Corrosion Protection in Carbon Steel †
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Materials
2.2. Development of Anticorrosive Pigments
2.3. Preparation of Carbon Steel Sample and Formulation of Coatings
2.4. Application of the Coatings on the Steel Sample
2.5. Characterization
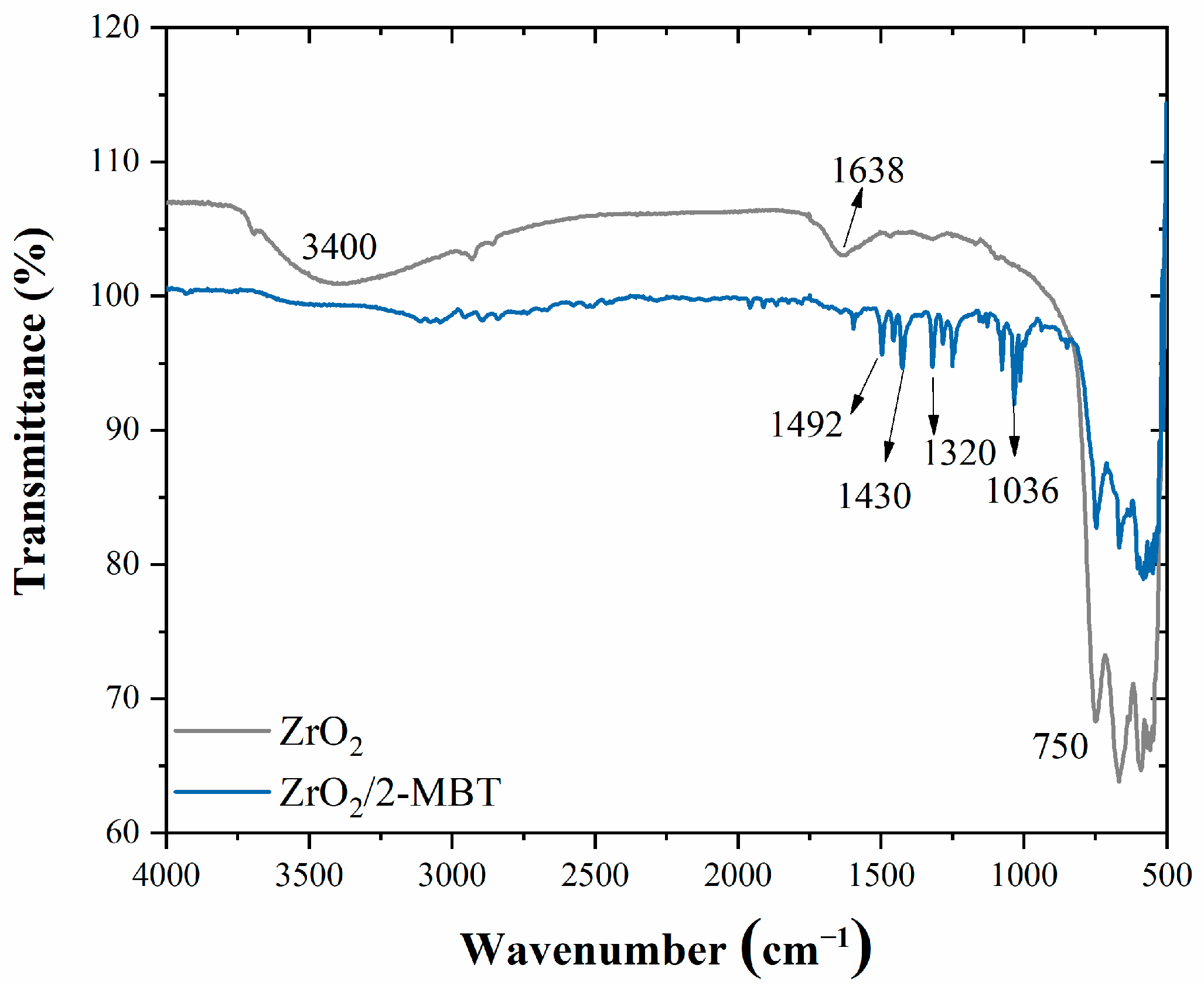
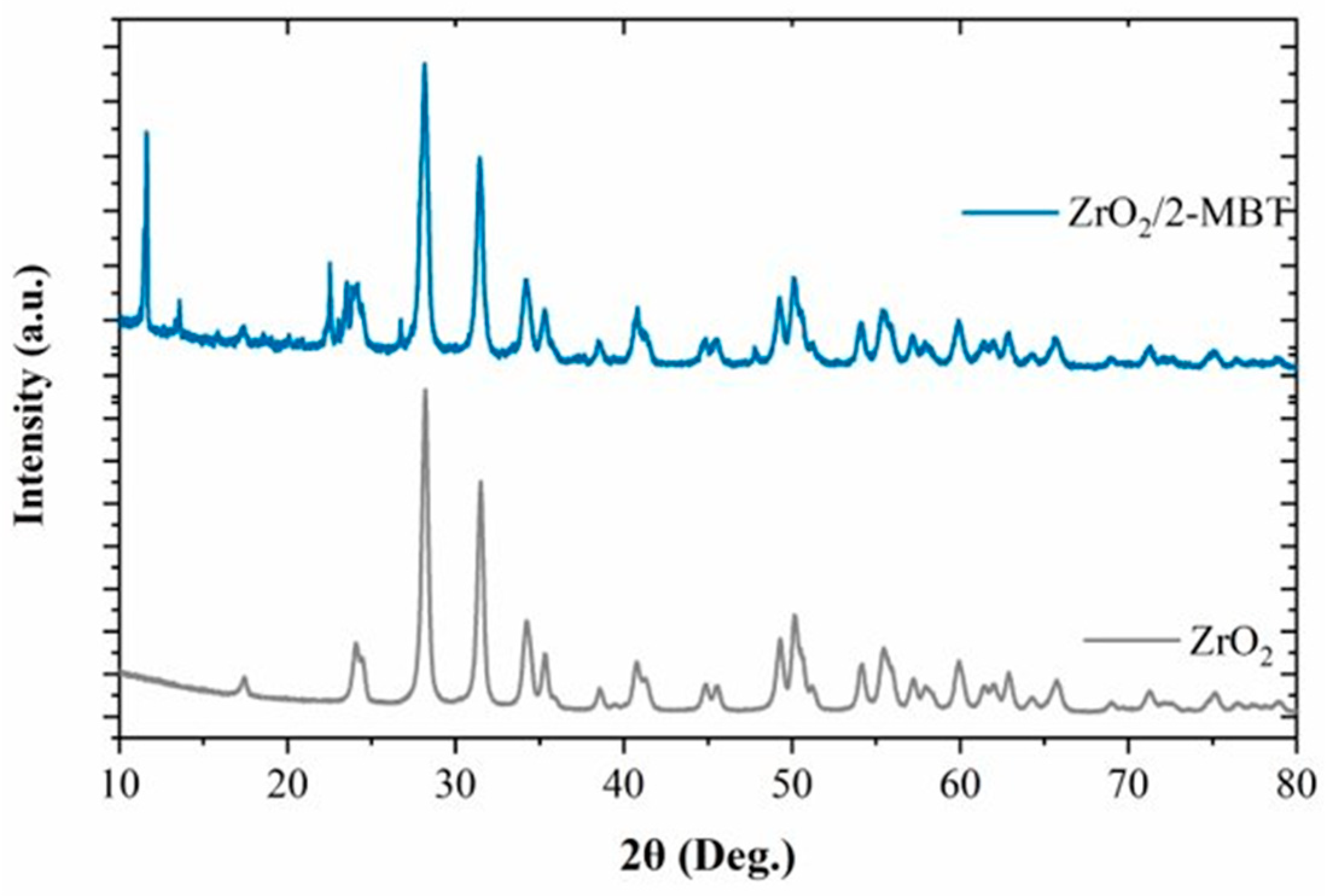
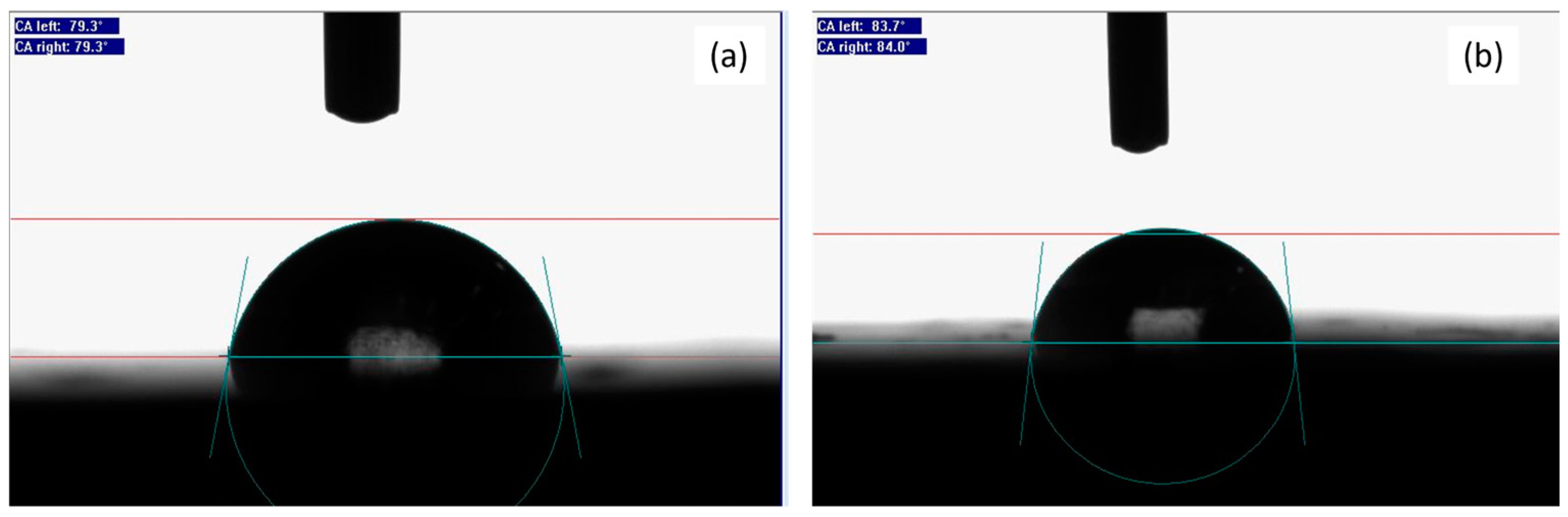
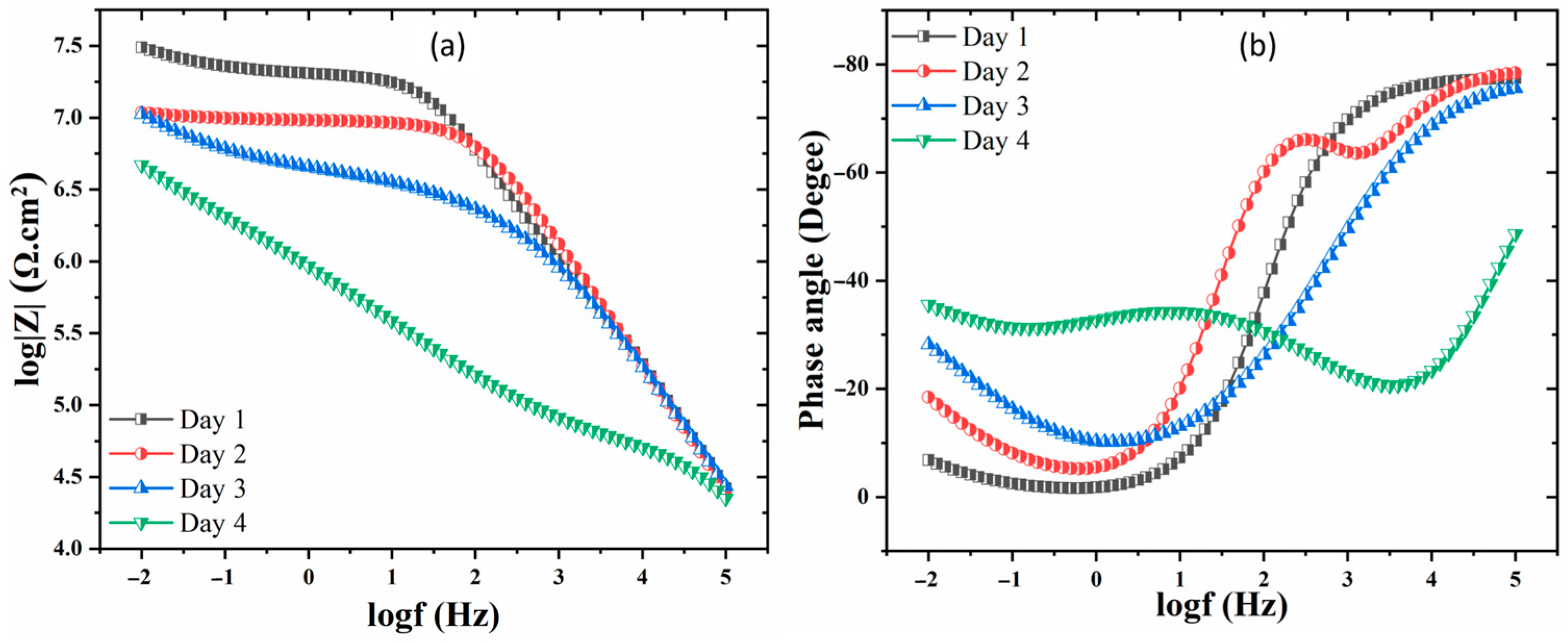
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Gurrappa, I.; Yashwanth, I.V.S. Yashwanth, Intelligent Coatings for Corrosion Control; Elsevier: Amsterdam, The Netherlands, 2015; pp. 17–58. [Google Scholar]
- Speight, J.G. Oil and Gas Corrosion Prevention; Butterworth-Heinemann: Oxford, UK, 2014; pp. 39–66. [Google Scholar]
- Morozov, Y.; Calado, L.M.; Shakoor, R.A.; Raj, R.; Kahraman, R.; Taryba, M.G.; Montemor, M.F. Epoxy Coatings Modified with a New Cerium Phosphate Inhibitor for Smart Corrosion Protection of Steel. Corr. Sci. 2019, 159, 108–128. [Google Scholar] [CrossRef]
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corr. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef]
- Bekas, D.G.; Tsirka, K.; Baltzis, D.; Paipetis, A.S. Self-healing materials: A review of advances in materials, evaluation, characterization and monitoring technique. Compos. B Eng. 2016, 87, 92–119. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Nawaz, M.; Radwan, A.B.; Kalambate, P.K.; Laiwattanapaisal, W.; Ubaid, F.; Akbar, H.M.; Shakoor, R.A.; Kahraman, R. Synergistic behavior of polyethyleneimine and epoxy monomers loaded in mesoporous silica as a corrosion-resistant self-healing epoxy coating. ACS Omega 2022, 7, 31700–31712. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.K.; Singh, R.; Singh, K.N.; Singh, V.B. FTIR, Raman spectra and ab initio calculations of 2-mercaptobenzothiazole. Spectrochim Acta A Mol. Biomol. Spectrosc. 2006, 63, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Dang, B.; Jin, T.; Chen, Y.; Zhang, J.; Feng, Q.; Liu, M.; Sun, Q. Effect of nano-zirconium oxide modification on flame retardancy of lignocellulose composite. Ind. Crops. Prod. 2022, 187, 115384. [Google Scholar] [CrossRef]
- Sherine, J.; Upadhyay, A.; Mishra, A.; Kumar, D.; Pal, S.; Harinipriya, S. Ag(I) and Au(III) Mercaptobenzothiazole complexes induced apoptotic cell death. Sci. Rep. 2019, 9, 621. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Meng, G.; Pershin, L.; Mostaghimi, J.; Coyle, T.W. Control of the hydrophobicity of rare earth oxide coatings deposited by solution precursor plasma spray by hydrocarbon adsorption. J. Mater. Sci. Technol. 2021, 62, 107–118. [Google Scholar] [CrossRef]
- Biswas, A.; Mourya, P.; Mondal, D.; Pal, S.; Udayabhanu, G. Grafting effect of gum acacia on mild steel corrosion in acidic medium: Gravimetric and electrochemical study. J. Mol. Liq. 2018, 251, 470–479. [Google Scholar] [CrossRef]
- Raj, R.; Taryba, M.G.; Morozov, Y.; Kahraman, R.; Shakoor, R.A.; Montemor, M.F. On the synergistic corrosion inhibition and polymer healing effects of polyolefin coatings modified with Ce-loaded hydroxyapatite particles applied on steel. Electrochim. Acta 2021, 388, 138648. [Google Scholar] [CrossRef]
- Njoku, D.I.; Cui, M.; Xiao, H.; Shang, B.; Li, Y. Understanding the anticorrosive protective mechanisms of modified epoxy coatings with improved barrier, active and self-healing functionalities: EIS and spectroscopic techniques. Sci. Rep. 2017, 7, 15597. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ani, M.A.; Al-Ardah, A.H.; Mahgoub, A.; Aboumattar, N.; Ibrahim, H.; Nawaz, M.; Shakoor, R.A.; Radwan, A.; Al-Qahtani, N. Intelligent Smart Coatings for Enhanced Corrosion Protection in Carbon Steel. Mater. Proc. 2024, 18, 1. https://doi.org/10.3390/materproc2024018001
Al-Ani MA, Al-Ardah AH, Mahgoub A, Aboumattar N, Ibrahim H, Nawaz M, Shakoor RA, Radwan A, Al-Qahtani N. Intelligent Smart Coatings for Enhanced Corrosion Protection in Carbon Steel. Materials Proceedings. 2024; 18(1):1. https://doi.org/10.3390/materproc2024018001
Chicago/Turabian StyleAl-Ani, Marwa A., Ala H. Al-Ardah, Amal Mahgoub, Noora Aboumattar, Hadir Ibrahim, Muddasir Nawaz, R. A. Shakoor, Ahmed Radwan, and Noora Al-Qahtani. 2024. "Intelligent Smart Coatings for Enhanced Corrosion Protection in Carbon Steel" Materials Proceedings 18, no. 1: 1. https://doi.org/10.3390/materproc2024018001
APA StyleAl-Ani, M. A., Al-Ardah, A. H., Mahgoub, A., Aboumattar, N., Ibrahim, H., Nawaz, M., Shakoor, R. A., Radwan, A., & Al-Qahtani, N. (2024). Intelligent Smart Coatings for Enhanced Corrosion Protection in Carbon Steel. Materials Proceedings, 18(1), 1. https://doi.org/10.3390/materproc2024018001








