Hydrometallurgical Recovery of Tin from Waste-Printed Circuit Boards †
Abstract
1. Introduction
2. Materials and Methods
2.1. Solid Characterization
2.2. Leaching of Tin
2.3. Chemical Precipitation of Tin
3. Results
3.1. Solid Characterization
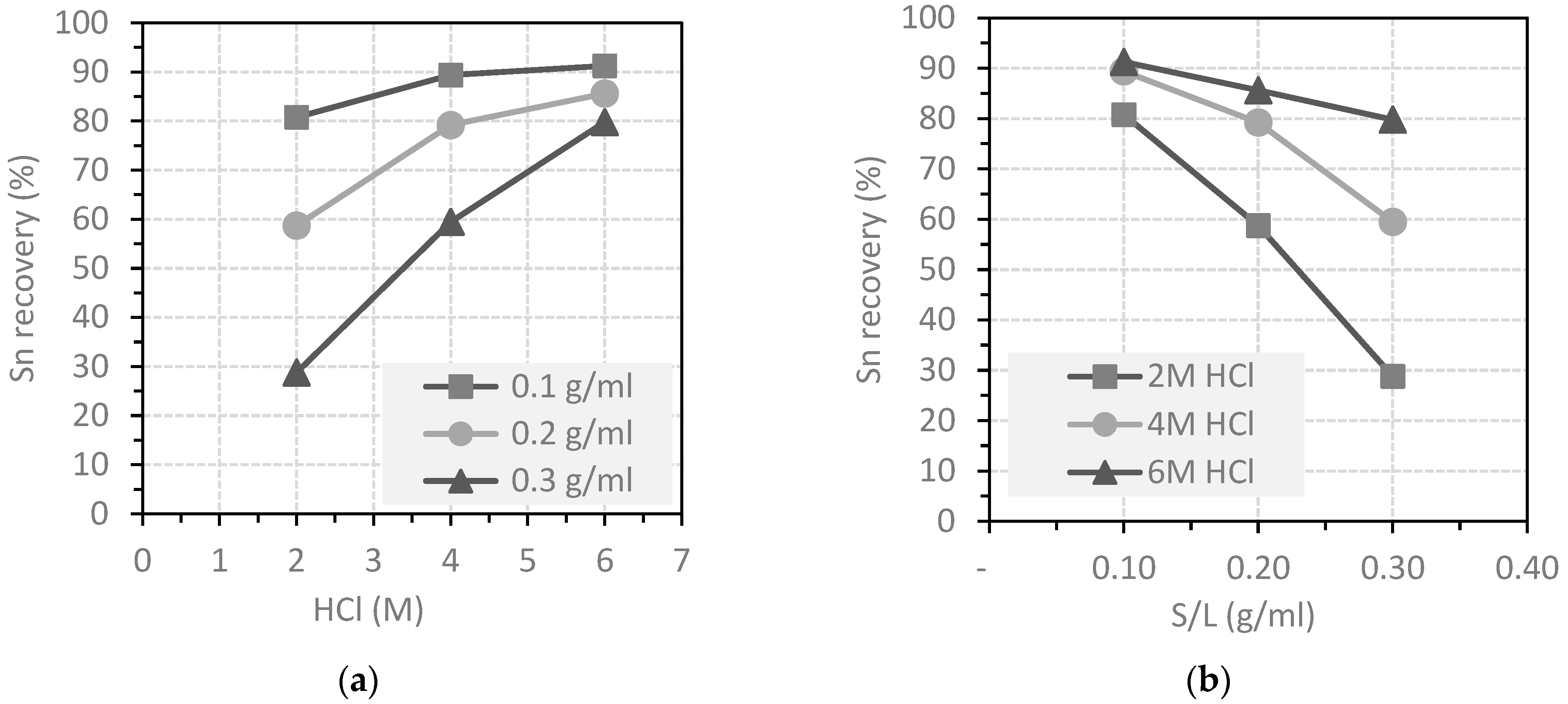
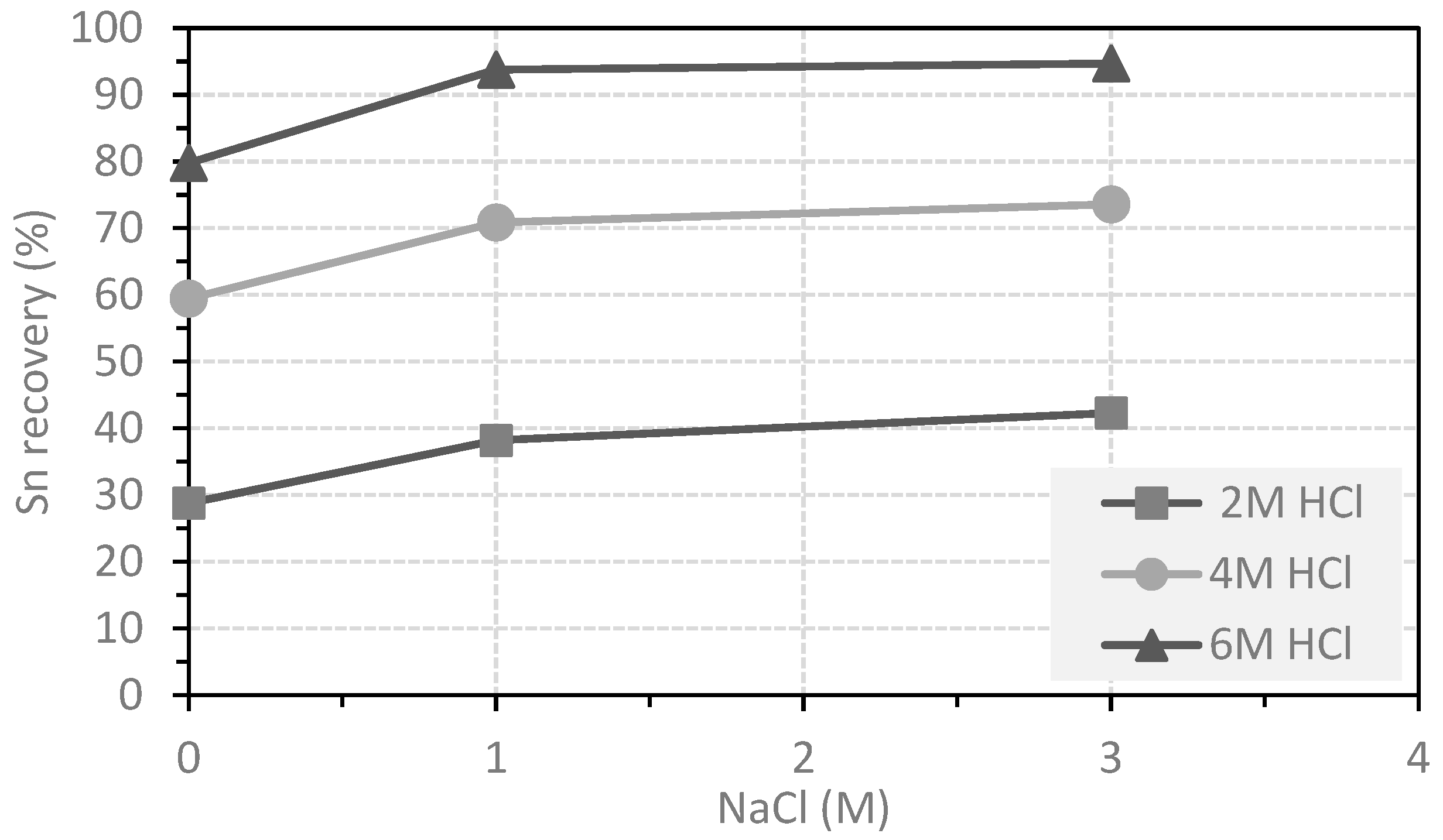
3.2. Leaching of Tin
- 6 M HCl concentration in the leaching solution;
- 3 M NaCl concentration in the leaching solution;
- S/L ratio of 0.3 g/mL;
- Residence time of 24 h;
- Room temperature;
- No agitation.
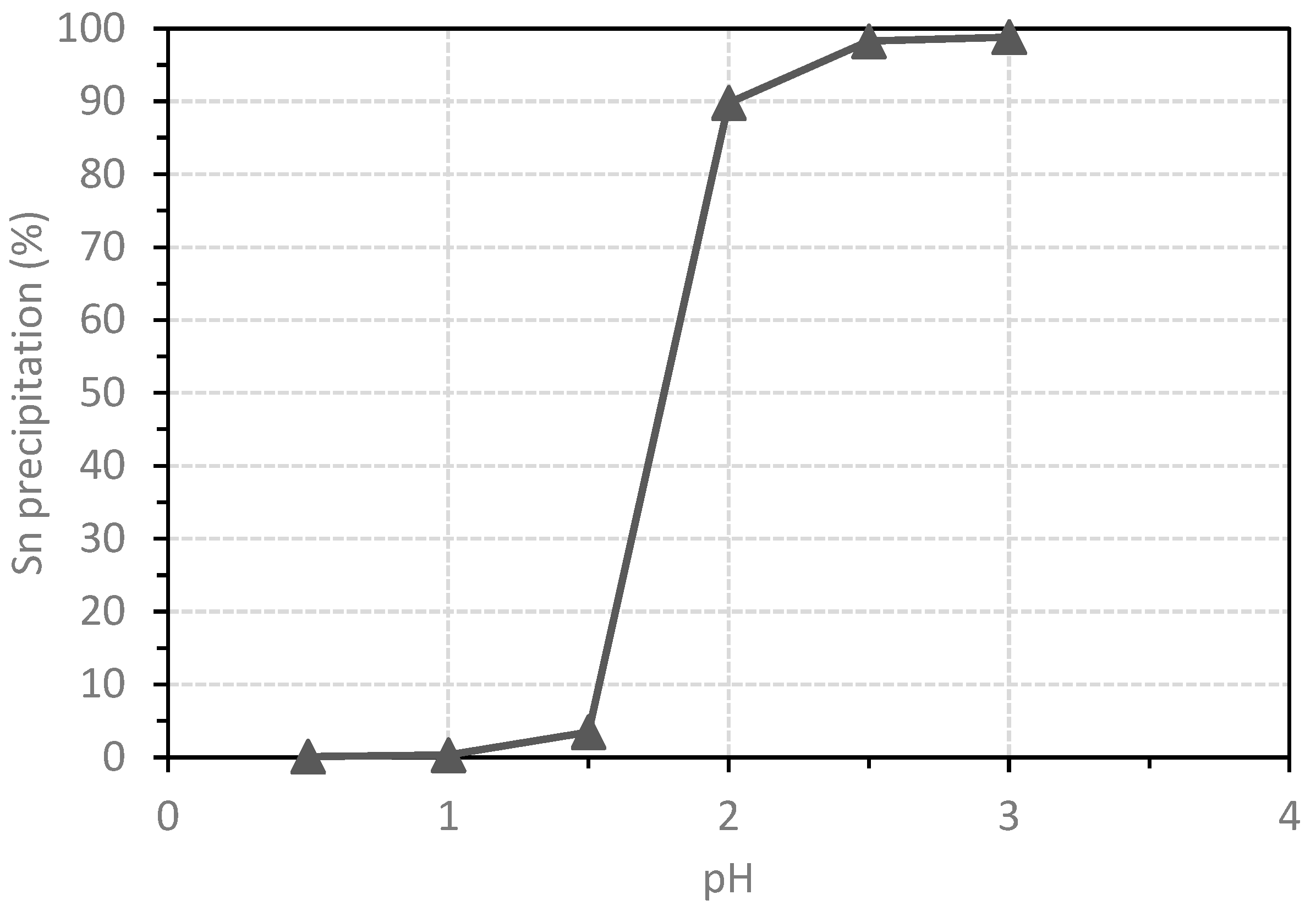
3.3. Chemical Precipitation of Tin
4. Conclusions
- The addition of NaCl in the leaching solution increases tin recovery to 95%.
- An increase in the S/L value reduces the tin dissolution efficiency.
- More than 95% of tin was precipitated from the pregnant solution at pH = 3.0.
- Tin content in the resulting precipitate exceeds 60% wt.
- Metal impurities in the precipitate did not exceed 10% wt.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Gao, Q.; Jiang, S.; Nie, C.; Zhu, X.; Jiao, T. Review on the gentle hydrometallurgical treatment of WPCBs: Sustainable and selective gradient process for multiple valuable metals recovery. J. Environ. Manag. 2023, 348, 119288. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.; Dhawan, N. A comprehensive review on the recycling of discarded printed circuit boards for resource recovery. Resour. Conserv. Recycl. 2022, 178, 106027. [Google Scholar] [CrossRef]
- Picazo-Rodriguez, N.G.; Baltierra-Costeira, G.; Soria-Aguilar, M.D.J.; Gamiño-Arroyo, Z.; Toro, N.; De la Garza de Luna, J.R.; Carrillo-Pedroza, F.R. E-waste Recycling: An Overview of Hydrometallurgical Processes Used to Metals Recovery. Preprints 2023, 110933. [Google Scholar] [CrossRef]
- Dutta, D.; Rautela, R.; Gujjala, L.K.S.; Kundu, D.; Sharma, P.; Tembhare, M.; Kumar, S. A review on recovery processes of metals from E-waste: A green perspective. Sci. Total Environ. 2023, 859, 160391. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eksteen, J.; Oraby, E. Hydrometallurgical recovery of metals from waste printed circuit boards (WPCBs): Current status and perspectives—A review. Resour. Conserv. Recycl. 2018, 139, 122–139. [Google Scholar] [CrossRef]
- Tunsu, C.; Retegan, T. Hydrometallurgical Processes for the Recovery of Metals from WEEE, Chapter 6. In Elsevier eBooks; Elsevier: Amsterdam, The Netherlands, 2016; pp. 139–175. [Google Scholar] [CrossRef]
| Metal | Initial Dust (% wt.) | Metallic Fraction after Separation (%wt.) |
|---|---|---|
| Sn | 5.01 | 12.74 |
| Cu | 5.19 | 23.86 |
| Fe | 8.17 | 17.84 |
| Zn | 1.90 | 3.36 |
| Ni | 0.76 | 1.48 |
| Pb | 1.53 | 6.28 |
| Al | 3.18 | 3.29 |
| Metal | Concentration (g/L) |
|---|---|
| Sn | 8.93 |
| Cu | 0.11 |
| Fe | 3.44 |
| Zn | 0.92 |
| Ni | 0.10 |
| Metal | Concentration (g/L) | |
|---|---|---|
| Before Precipitation | After Precipitation at pH = 3.0 | |
| Sn | 7.44 | 0.09 |
| Cu | 0.09 | 0.05 |
| Fe | 2.87 | 2.53 |
| Zn | 0.77 | 0.63 |
| Ni | 0.08 | 0.08 |
| Metal | Precipitate (% wt.) |
|---|---|
| Sn | 60.25 |
| Cu | 0.65 |
| Fe | 6.28 |
| Zn | 2.73 |
| Ni | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasopoulos, D.; Oustadakis, P.; Remoundaki, E.; Agatzini-Leonardou, S. Hydrometallurgical Recovery of Tin from Waste-Printed Circuit Boards. Mater. Proc. 2023, 15, 90. https://doi.org/10.3390/materproc2023015090
Vlasopoulos D, Oustadakis P, Remoundaki E, Agatzini-Leonardou S. Hydrometallurgical Recovery of Tin from Waste-Printed Circuit Boards. Materials Proceedings. 2023; 15(1):90. https://doi.org/10.3390/materproc2023015090
Chicago/Turabian StyleVlasopoulos, Dimitrios, Paschalis Oustadakis, Emmanouella Remoundaki, and Styliani Agatzini-Leonardou. 2023. "Hydrometallurgical Recovery of Tin from Waste-Printed Circuit Boards" Materials Proceedings 15, no. 1: 90. https://doi.org/10.3390/materproc2023015090
APA StyleVlasopoulos, D., Oustadakis, P., Remoundaki, E., & Agatzini-Leonardou, S. (2023). Hydrometallurgical Recovery of Tin from Waste-Printed Circuit Boards. Materials Proceedings, 15(1), 90. https://doi.org/10.3390/materproc2023015090






