Composite Lightweight Materials with Upgraded Physicochemical Functionality and Improved Economic Feasibility †
Abstract
1. Introduction
2. Materials and Methods
2.1. Perlite Substrates
2.2. Perlite-Chitosan BioComposites: Fabrication—Batch Absorption Studies
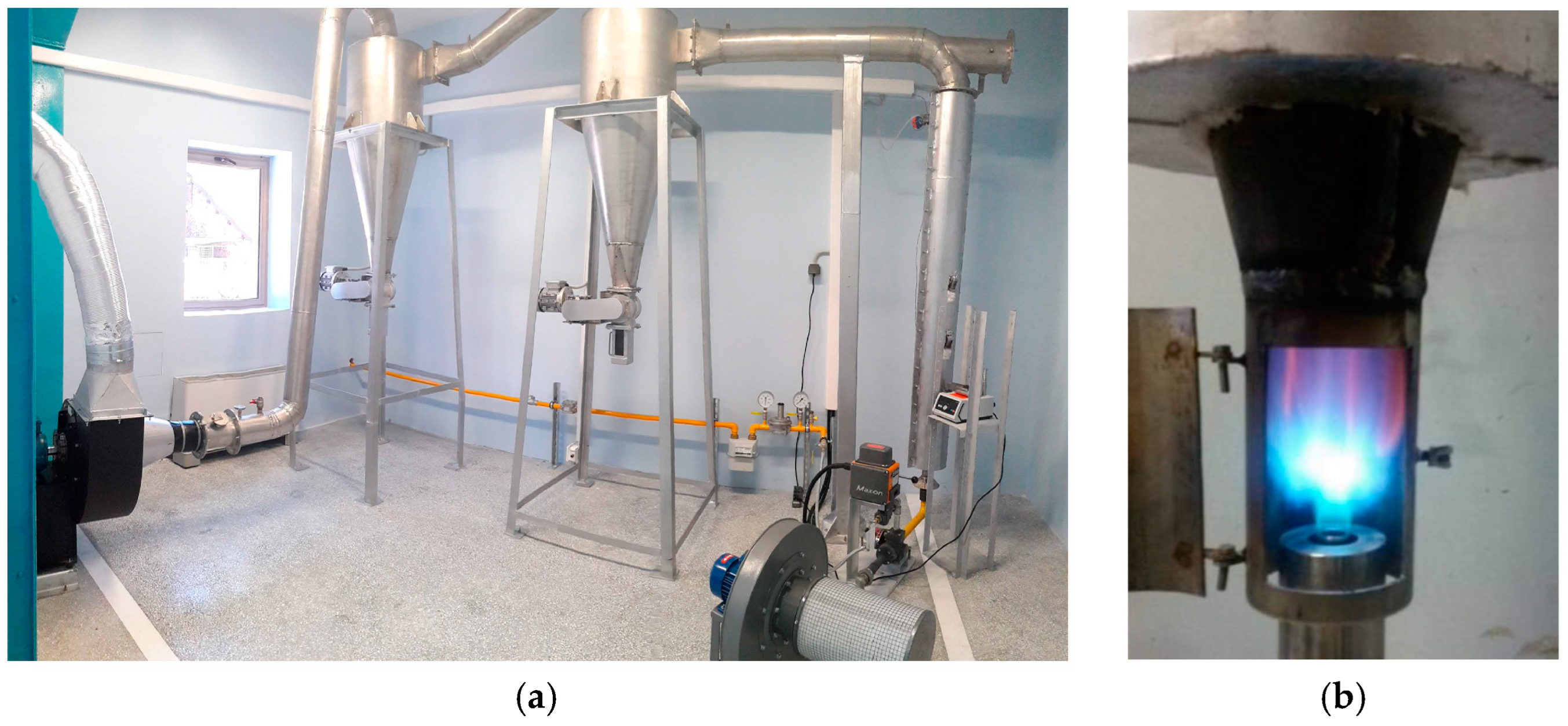
2.3. Photocatalytic Composites: Fabrication—Characterisation of Photocatalytic Properties
2.4. Perlite–PCM Composites: Fabrication—Mortar Preparation
3. Results and Discussion
3.1. Perlite Substrates
3.2. Perlite–Chitosan Biocomposites—Batch Absorption Studies
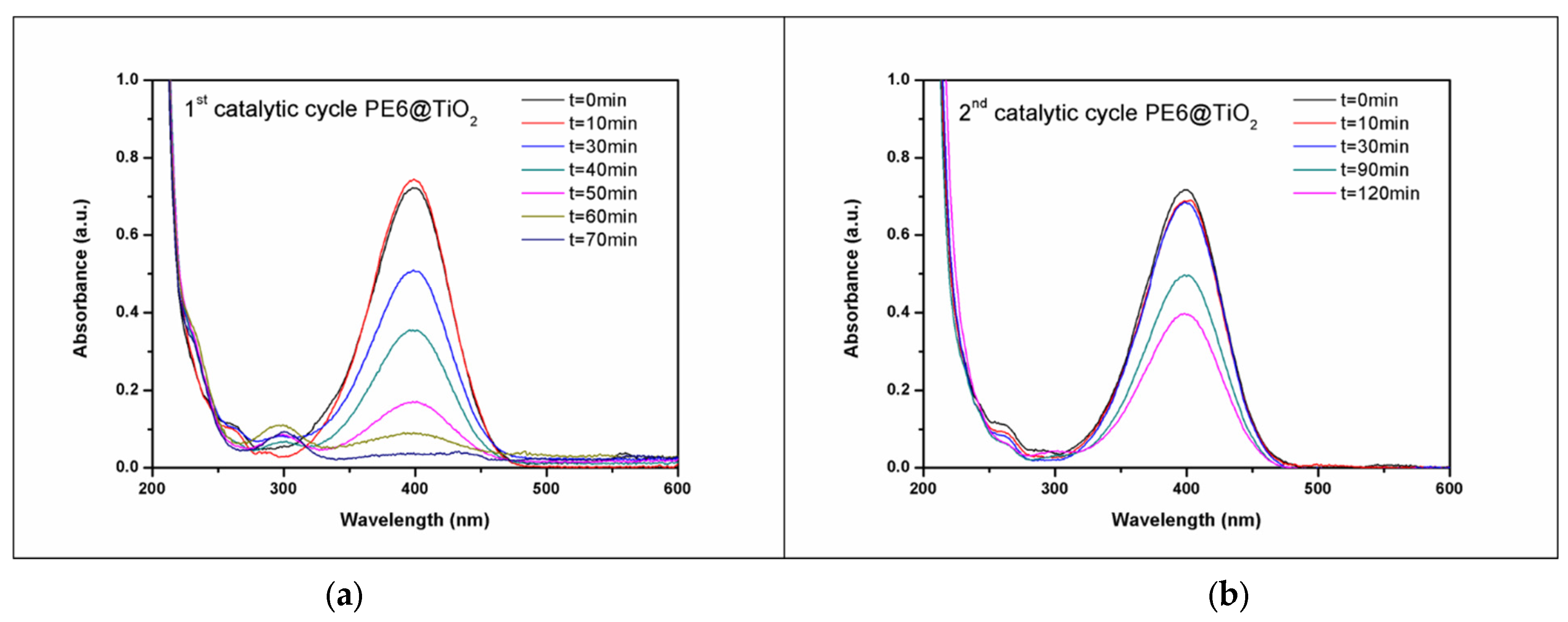
3.3. Photocatalytic Composites
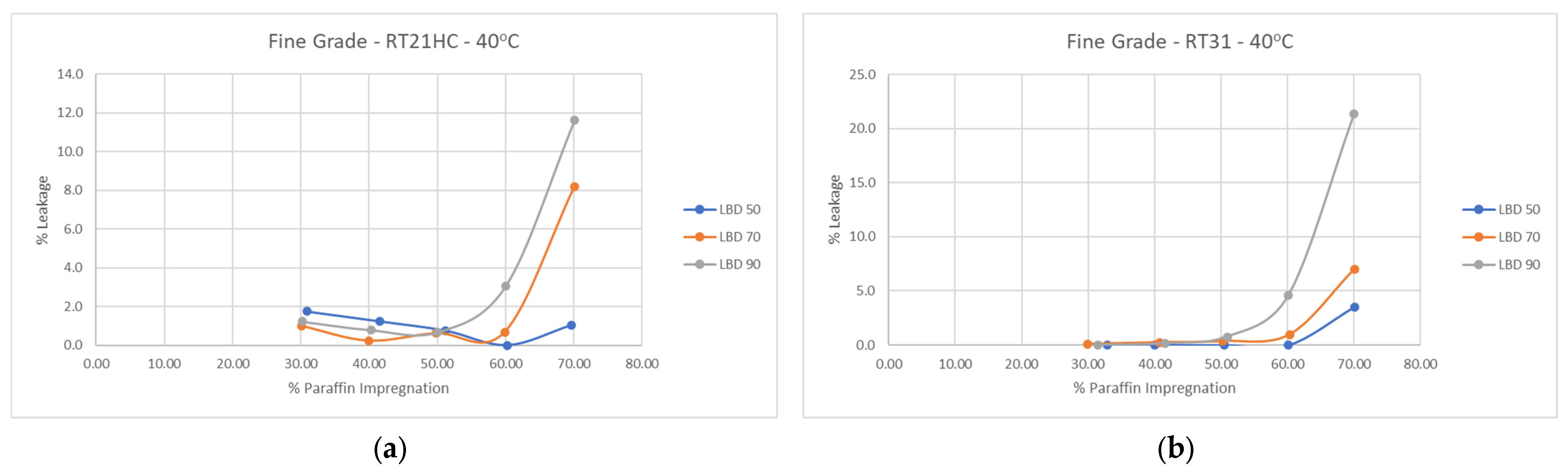
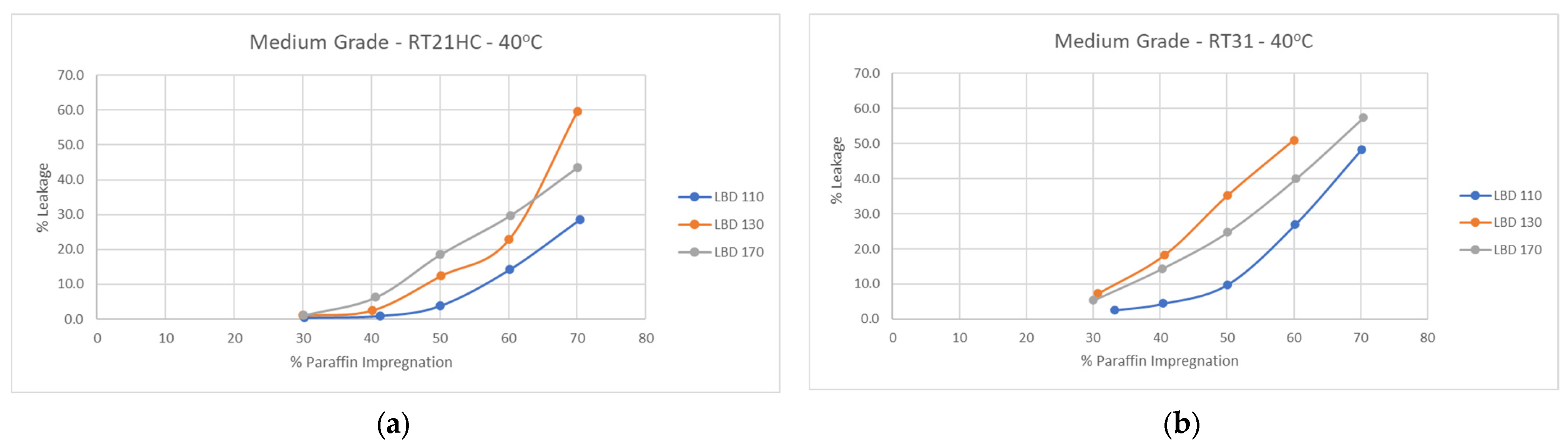
3.4. Perlite–PCM Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasan, S.; Krishnaiah, A.; Ghosh, T.K.; Viswanath, D.S.; Boddu, V.M.; Smith, E.D. Adsorption of Chromium(VI) on Chitosan-Coated Perlite. Sep. Sci. Technol. 2003, 38, 3775–3793. [Google Scholar] [CrossRef]
- Ngah, W.W.; Teong, L.; Hanafiah, M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Sanjayan, J.; Wang, X.; Alam, M.; Wilson, J. A novel paraffin/expanded perlite composite phase change material for prevention of PCM leakage in cementitious composites. Appl. Energy 2015, 157, 85–94. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Feng, T.; Zhou, M.; Zhao, L.; Zhou, A.; Li, Z. Immobilizing bacteria in expanded perlite for the crack self-healing in concrete. Constr. Build. Mater. 2017, 148, 610–617. [Google Scholar] [CrossRef]
- Peng, K.; Yang, H.M. Preparation of Aerogel-Modified Expanded Perlite and its Application in Heat Insulation Coating. Adv. Mater. Res. 2013, 668, 360–364. [Google Scholar] [CrossRef]
- Xue, H.; Jiang, Y.; Yuan, K.; Yang, T.; Hou, J.; Cao, C.; Feng, K.; Wang, X. Floating photocatalyst of B–N–TiO2/expanded perlite: A sol–gel synthesis with optimized mesoporous and high photocatalytic activity. Sci. Rep. 2016, 6, 29902. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, S.; Krishnaiah, A.; Boddu, V.M. Adsorption of Divalent Cobalt from Aqueous Solution onto Chitosan–Coated Perlite Beads as Biosorbent. Sep. Sci. Technol. 2007, 42, 2767–2786. [Google Scholar] [CrossRef]
- Hasan, S.; Ghosh, T.K.; Viswanath, D.S.; Boddu, V.M. Dispersion of chitosan on perlite for enhancement of copper(II) adsorption capacity. J. Hazard. Mater. 2007, 152, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Blaskov, V.; Stambolova, I.; Georgiev, V.; Batakliev, T.; Eliyas, A.; Shipochka, M.; Vassilev, S.; Mehandjiev, D. Synthesis and Catalytic Activity of Silver-Coated Perlite in the Reaction of Ozone Decomposition. Ozone Sci. Eng. 2014, 37, 252–256. [Google Scholar] [CrossRef]
- Pilatos, G.; Samouhos, M.; Angelopoulos, P.; Taxiarchou, M.; Veziri, C.; Hutcheon, R.; Tsakiridis, P.; Kontos, A.G. Carbon nanotubes growth on expanded perlite particles via CVD method: The influence of the substrate morphology. Chem. Eng. J. 2016, 291, 106–114. [Google Scholar] [CrossRef]
- Swayampakula, K.; Boddu, V.M.; Nadavala, S.K.; Abburi, K. Competitive adsorption of Cu (II), Co (II) and Ni (II) from their binary and tertiary aqueous solutions using chitosan-coated perlite beads as biosorbent. J. Hazard. Mater. 2009, 170, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Kitsou, I.; Panagopoulos, P.; Maggos, T.; Arkas, M.; Tsetsekou, A. Development of SiO2@TiO2 core-shell nanospheres for catalytic applications. Appl. Surf. Sci. 2018, 441, 223–231. [Google Scholar] [CrossRef]
- Gangula, A.; Podila, R.; Ramakrishma, M.; Karanam, L.; Janardhana, C.; Rao, A.M. Catalytic Reduction of 4-Nitrophenol using Biogenic Gold and Silver Nanoparticles Derived from Breynia rhamnoides. Langmuir 2011, 27, 15268–15274. [Google Scholar] [CrossRef] [PubMed]
- Aditya, T.; Pal, A.; Pal, T. Nitroarene reduction: A trusted model reaction to test nanoparticle catalysts. Chem. Commun. 2015, 51, 9410–9431. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wu, D.; Zhang, S.; Zhang, L.; Hu, W.; Zhu, C.; Gong, X. Effective photodegradation of 4-nitrophenol with CuO nano particles prepared by ionic liquids/water system. Green Chem. Eng. 2022, 3, 15–24. [Google Scholar] [CrossRef]
- Yadav, V.; Verma, P.; Negi, H.; Singh, R.K.; Saini, V.K. Efficient degradation of 4-nitrophenol using VO(TPP) impregnated TiO2 photocatalyst: Insight into kinetics and mechanism. J. Mater. Res. 2023, 38, 237–247. [Google Scholar] [CrossRef]

| Grade/Substrate Codes | Raw Grade (Particle Size) | LBD (kg/m3) | Biocomposite Codes |
|---|---|---|---|
| Ultrafine—Microspheres/UF-257 | −0.063 mm | 257 | BCUF-257 |
| Fine Grade/F-90 | 0.3/0.075 mm | 90 | BCF-90 |
| Medium Grade/M-110 | 1.18/0.5 mm | 110 | BCM-110 |
| Medium Grade/M-170 | 1.18/0.5 mm | 170 | BCM-170 |
| Grade/Substrate Codes | Raw Grade (Particle Size) | LBD (kg/m3) | Photo-Composite Codes |
|---|---|---|---|
| Ultrafine—Microspheres/UF-222 | −0.063 mm | 222 | PE4@TiO2 |
| Ultrafine—Microspheres/UF-257 | −0.053 mm | 257 | PE6@TiO2 |
| Substrate Code | LBD (kg/m3) | Water Load (mLH2O/gperl) | Oil Absorption (g oil/g Perlite) | Compression Resistance (Vol Reduction) | Skeletal Density (kg/m3) |
|---|---|---|---|---|---|
| M-110 | 95.9 | 2.07 | 2.93 | 72.1% | 822.3 |
| M-130 | 123.0 | 2.09 | 2.16 | 62.2% | 426.6 |
| M-170 | 167.8 | 1.61 | 1.85 | 41.4% | 508.9 |
| Substrate Code | LBD (kg/m3) | Water Load (mLH2O/gperl) | Oil Absorption (g oil/g Perlite) | Compression Resistance (Vol Reduction) | Skeletal Density (kg/m3) |
|---|---|---|---|---|---|
| F-50 | 46.9 | 9.36 | 5.48 | 72.1% | 822.3 |
| F-70 | 67.4 | 6.45 | 4.45 | 62.2% | 426.6 |
| F-90 | 89.4 | 4.67 | 3.28 | 55.9% | 439.2 |
| F-120 | 119.8 | 3.66 | 2.40 | 45.6% | 484.6 |
| F-140 | 141.3 | 3.21 | 2.06 | 41.4% | 508.9 |
| Substrate Code | Particle Size | LBD (kg/m3) | Oil Absorption (g oil/g Perlite) | Compression Resistance (Vol Reduction) | Skeletal Density (kg/m3) |
|---|---|---|---|---|---|
| UF-222 | −0.063 mm | 222.2 | 1.17 | 34.6% | 1214.2 |
| UF-257 | −0.053 mm | 256.5 | 0.94 | 30.9% | 1237.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simos, X.; Papageorgiou, M.; Kitsou, I.; Mamasi, M.E.; Gikarakis, T.; Ekonomakou, A.; Amanatidis, A.; Anastassakis, G.N.; Tsetsekou, A. Composite Lightweight Materials with Upgraded Physicochemical Functionality and Improved Economic Feasibility. Mater. Proc. 2023, 15, 84. https://doi.org/10.3390/materproc2023015084
Simos X, Papageorgiou M, Kitsou I, Mamasi ME, Gikarakis T, Ekonomakou A, Amanatidis A, Anastassakis GN, Tsetsekou A. Composite Lightweight Materials with Upgraded Physicochemical Functionality and Improved Economic Feasibility. Materials Proceedings. 2023; 15(1):84. https://doi.org/10.3390/materproc2023015084
Chicago/Turabian StyleSimos, X., M. Papageorgiou, I. Kitsou, M. E. Mamasi, T. Gikarakis, A. Ekonomakou, A. Amanatidis, G. N. Anastassakis, and A. Tsetsekou. 2023. "Composite Lightweight Materials with Upgraded Physicochemical Functionality and Improved Economic Feasibility" Materials Proceedings 15, no. 1: 84. https://doi.org/10.3390/materproc2023015084
APA StyleSimos, X., Papageorgiou, M., Kitsou, I., Mamasi, M. E., Gikarakis, T., Ekonomakou, A., Amanatidis, A., Anastassakis, G. N., & Tsetsekou, A. (2023). Composite Lightweight Materials with Upgraded Physicochemical Functionality and Improved Economic Feasibility. Materials Proceedings, 15(1), 84. https://doi.org/10.3390/materproc2023015084





