Abstract
The present research work focuses on the characterization and leachability evaluation of electric arc furnace dust (EAFD) and its vitreous outgrowths produced during vitrification with soda lime recycled glass (SLRG). EAFD is a hazardous industrial waste generated in the collection of particulate material during the steelmaking process via an electric arc furnace. Glasses of various syntheses were obtained during EAFD vitrification with various amounts of silica scrap (50, 60 and 70 wt%). The characterization of the as-received dust was carried out by using granulometry analysis, chemical analysis, X-ray diffraction (XRD), and scanning electron microscopy (SEM), in conjunction with Energy Dispersive X-ray Spectroscopy (EDS). The produced glasses during vitrification were studied by means of chemical and mineralogical analysis, whereas their microstructure in polished sections was examined by SEM/EDS. Their behavior during leaching was determined by the EN 12457-2 compliance leaching test and according to the results, the trace elements detected in the leachates were well below the corresponding regulatory limits.
1. Introduction
Electric arc furnace dust (EAFD) is a hazardous waste generated in the steelmaking industry during the production of steel in electric arc furnaces. It consists of fine particles that are collected from the flue gases of the furnace. During steel scrap melting, various evaporated components are condensed and removed together with the rest of particulate material in the off-gas cleaning system [1,2]. The temperature of the EAF usually varies from 1600 °C to 1700 °C, or even higher, and various phases from the scrap feed, including Fe, Zn and Pb, are evaporated and enter the gas stream. The largest part of EAFD is produced during vapor stream cooling and it is usually collected with bag filters.
EAFD is primarily composed of Fe, Zn, Pb oxides and other heavy metals. The exact composition depends on the feed materials used in the steelmaking process. When galvanized recycled scrap is fed to the EAF, almost all of the Zn from the steel coatings is volatized in the gas fume, since its evaporation is carried out at 950 °C. Also, its solubility in both molten steel and slag is very low. As a result, Zn vapor can react with other evaporated compounds producing various mixed phases, such as zinc oxides and zinc ferrites. It should be noted that the Zn content in EFAD can exceed 40%, mainly depending on the initial proportion of galvanized scrap of the feed. Other hazardous heavy metals such as Cd, Cr and As may also be present [3,4].
The electric furnace dust production corresponds to 15–20 kg per ton of recycled iron scrap. The annual world EAFD production reaches 4 million tons. Europe steelmaking plants produce about 500.000–900.000 tons of EAFD every year [5]. In Greece, the corresponding EAFD production from the scrap-recycling steelmaking plants is estimated at about 15.000–20.000 tons per year [6]. It is known that EAFD has been classified as hazardous waste according to the European Waste Catalogue (EWC 2002), mainly because of its chemical and physical properties [7]. Consequently, its management demands specific handling and disposal methods (stabilization/solidification) in order to reduce the environmental impact. The present research work focuses on the characterization and leachability evaluation of both electric arc furnace dust and its vitreous outgrowths, produced during vitrification with soda lime recycled glass (SLRG).
2. Materials and Methods
EAFD was collected from a dust-collecting system of a steelmaking operation in Greece. Three different vitreous outgrowths were developed, by substituting the initial EAFD by SLRG for 70 wt% (VEAFD-30), 60 wt% (VEAFD-40) and 50 wt% (VEAFD-50). Chemical analyses (Table 1) of both raw materials (EAFD and SLRG), along with the corresponding vitrified outgrowths, were performed via X-ray Fluorescence (Spectro–Xepos, Kleve. Germany), Atomic Absorption Spectrophotometry (Perkin-Elmer 4100, PerkinElmer Inc., Hopkinton, MA, USA) and Inductively Coupled Plasma Mass Spectrometry (ICP-MS X Series II, Thermo Fisher Scientific, Courtaboeuf, France).

Table 1.
Chemical analyses of the raw materials and vitreous outgrowths.
EAFD was vitrified at 1200 °C, for 1 h, by using a mixture of green and brown/amber SLRG gathered from a local municipal waste recycling plant. SLRG consists of silica and various amounts of sodium and calcium carbonates (Na2CO3, CaCO3) whose addition as fluxes is required in order to decrease the final melting point. Various vitreous glasses were developed by substituting the original EAFD by SLRG for 50, 60 and 70 wt%. The initial raw mixtures were grinded in a ball mill to achieve a final gain size lower than 90 μm. Particle size distribution was determined by a laser scattering particle size distribution analyzer (Mastersizer 2000, Malvern Instruments Ltd., Worcestershire, UK) after dispersion treatment by an ultrasonic homogenizer. The final samples were vitrified at 1200 °C for approximately 1 h. X-ray diffraction (XRD) was performed for phase identification using a Bruker D8-Focus diffractometer (Bruker AXS, Karlsruhe, Germany) with nickel-filtered Cu Ka radiation (λ = 1.5405 Å) at 40 kV and 40 mA. The morphology of the initial EAFD and vitreous products was examined by scanning electron microscopy (SEM) using a Jeol 6380LV Scanning Electron Microscope (JEOL, Tokyo, Japan), in conjunction with an Oxford INCA (Oxford Instruments, Abingdon, UK) Energy Dispersive Spectrometer (EDS) connected to the SEM.
Both EAFD and its glasses produced via vitrification were further subjected to leachability evaluation through the batch test EN 12457-2. The test was performed in a batch reactor by mixing the raw material with deionized water in a solid/liquid ratio of 1:10 and kept under continuous stirring (10 rpm) for 24 h at a controlled temperature (20 ± 5 °C). The values of dissolved elements were compared with the regulatory limit values issued by the European Council [8].
3. Results and Discussion
Table 1 presents the results of EAFD chemical analysis, which mainly depends on both the initial recycled iron scrap quality used and the final steel category being developed. Besides Fe and Zn, Ca was also present in a high amount mainly due to the lime added during the steelmaking process. EAFD and SLRG initial syntheses were co-grinded in a ball mill, and their corresponding particle size distributions are shown in Figure 1, together with that of the initial EAFD. It was found that in all cases, 50% of mixture particles were under 23 μm, while 90% of the particles were below 63 μm.
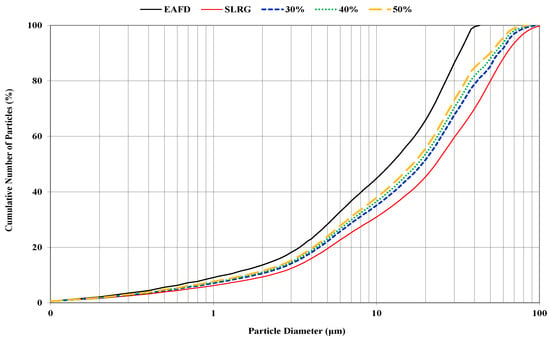
Figure 1.
Particle size distributions of the EAFD and raw mixtures after grinding.
The mineralogical composition of both raw materials and raw mixtures is presented in Figure 2, whereas the corresponding SEM micrographs of the as-received EAFD are shown in Figure 3. Two major phases were identified: zincite and franklinite. Zinc in the as-received EAFD is in the forms of zincite (ZnO) and franklinite (ZnFe2O4), while Fe has been bound mainly in ZnFe2O4. Furthermore, other phases such as portlandite, lime, and calcite (Ca(OH)2, CaO, CaCO3), halite (NaCl), lead oxide and cerussite (PbO, PbCO3), CaMgSiO4 and CaAl4Fe8O19 are also present as minor constituents. The amorphous nature of SLRG was attested by the detection of a diffuse wide band coming from the glassy phase in the range of 15−40°, generated during rapid cooling. Regarding the EAFD morphological characteristics, both spherical fine-grained and elongated particles were detected (Figure 3). Agglomeration phenomena were predominant, as finer particles accumulate, developing aggregates or masking other larger particles. EDS analyses certified the existence of zinc and iron, while smaller amounts of calcium, lead, magnesium and chromium were also detected.
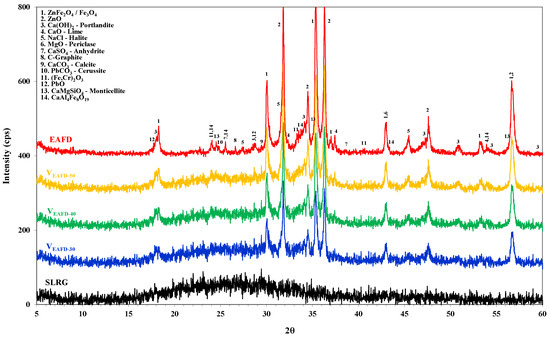
Figure 2.
Mineralogical phases of the EAFD, SLRG and raw mixtures.
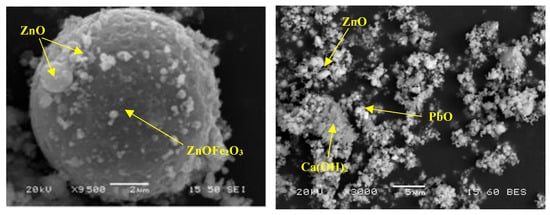
Figure 3.
SEM micrographs of the as-received EFAD.
The vitrification process was carried out by replacing 30, 40 and 50 wt% of the EAFD with SLRG. The chemical analysis results of the vitreous outgrowths after sintering are given in Table 1. The mineralogical analysis results, obtained via X-ray diffraction, are presented in the corresponding patterns in Figure 4. In the case of 70 wt% EAFD substitution, the glassy matrix is predominant, whereas as the EAFD content is increased, three major crystalline phases were detected: spinel (Fe,Mn,Zn)(Fe,Mn)2O4, augite (Ca(Mg,Fe,Al)(Si,Al)2O6) and diopside (CaMg(SiO3)2). Spinel was absent in the case of VEAFD30, whereas it was one of the dominant crystalline phases in case of lower SLRG content (Fe and Zn content increment). Augite is a pyroxene mineral incorporating a wide range of cations. In the case of the CaO-MgO-Al2O3-SiO2 glass system, augite and diopside (both clinopyroxenes) are normally the main crystal phases. Diopside is the end-member clinopyroxene, while augite is the main phase.
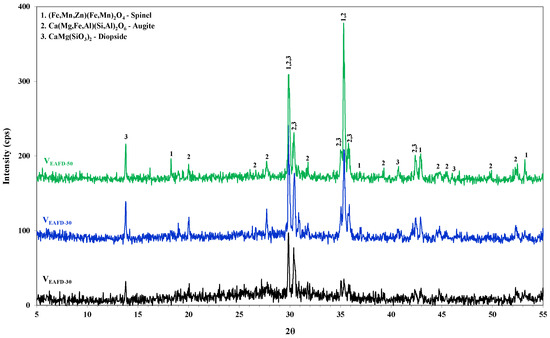
Figure 4.
Mineralogical phases of the produced vitreous outgrowths.
The above results were also confirmed under an electron microscope in polished sections (Figure 5). During vitrification, the transformation phenomena and the consequent crystallization evolved in two stages: nucleation and growth. During nucleation, slight contiguous nucleuses started to develop, mostly at preferred sites. Zinc ferrite (spinel phase) developed, via nucleation from the melt, idiomorphic grains, but is also present in eutectic growth with augite. These types of spinel phases are amongst the first to nucleate from the glass melt. While the silicates nucleate simultaneously with the oxides, small crystals of spinel appeared to be embedded in the augite phase. Clinopyroxenes (augite and diopside) were detected with the form of euhedral prismatic long crystals. Both augite and diopside accumulating Ca, coming from the soda lime glass, were developed in the glass matrix mainly due to a relatively slow rate of cooling, which allowed the formation of euhedral crystals. The amorphous glass matrix has been observed to be colorless, presenting high elemental homogeneity, since it has been developed mostly by silicon, sodium, calcium, zinc iron and lead.
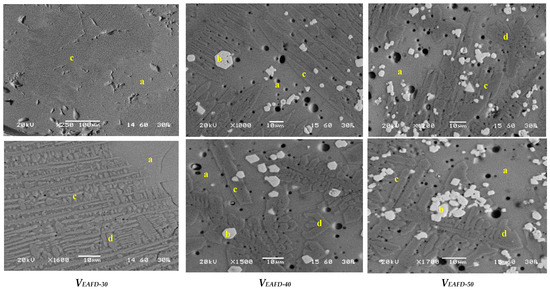
Figure 5.
Backscattered electron micrographs of vitreous outgrowths polished sections. a: amorphous glass, b: spinel (Fe,Mn,Zn)(Fe,Mn)2O4, c: augite (Ca(Mg,Fe,Al)(Si,Al)2O6); d: diopside (CaMg(SiO3)2.
Table 2 presents the leachability metals values of EAFD and those of the corresponding vitreous products, according to the EN12457-2 leaching test. Regarding EAFD, the leaching values for lead and zinc were significantly higher than the corresponding accepted limits for hazardous wastes, while after the proposed sintering process with SLRG, no heavy metals were observed after water leaching for 24 h at 25 °C. The final leach liquor pH after water leaching treatment, in all cases, was measured in the range of 7-7.5, while no precipitates were observed.

Table 2.
Released metal concentration after EN 12457-2 leaching test at L/S = 10 L/kg.
4. Conclusions
Electric arc furnace dust (EAFD) was vitrified at 1200 °C with soda lime recycled glass (SLRG). Various different vitreous glasses were developed via substituting EAFD with silica scrap for 50, 60 and 70 wt%. In the case of 70 wt% EAFD substitution, the glassy matrix was predominant, whereas as the EAFD content increased, three major crystalline phases were detected: spinel (Fe,Mn,Zn)(Fe,Mn)2O4, augite (Ca(Mg,Fe,Al)(Si,Al)2O6) and diopside (CaMg(SiO3)2). Clinopyroxenes (augite and diopside) were detected with the form of euhedral prismatic long crystals. On the other hand, zinc ferrite (spinel phase) was developed through nucleation from the melt into idiomorphic grains, but was also detected in eutectic growth with augite. The leaching behavior of the original EAFD according to the EN 12457-2 compliance test confirmed its hazardous nature, as the leaching values for lead and zinc ions were found to be significantly higher than the accepted limits for hazardous wastes, according to the 2003/33/EC directive. On the other hand, no heavy metals were observed in the produced glassy outgrowth leach liquors.
Author Contributions
All authors contributed to the study conception, material preparation, and data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not relevant.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting the reported results have been included in the manuscript.
Conflicts of Interest
No conflicts of interest.
References
- Barrett, E.C.; Nenniger, E.H.; Dziewinski, J. A hydrometallurgical process to treat carbon steel electric arc furnace dust. Hydrometallurgy 1992, 30, 59–68. [Google Scholar] [CrossRef]
- Martins, F.M.; Neto, J.M.R.; Cunha, C.J. Mineral phases of weathered and recent electric arc furnace dust. J. Hazard. Mater. 2008, 154, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Salihoglu, G.; Pinarli, V. Steel foundry electric arc furnace dust management: Stabilization by using lime and Portland cement. J. Hazard. Mater. 2008, 153, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.F.; Galiano, Y.L.; Rodríguez-Piñero, M.A.; Parapar, J.V. Long and short-term performance of a stabilized/solidified electric arc furnace dust. J. Hazard. Mater. 2007, 148, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.G.M.S.; Brehm, F.A.; Moraes, C.A.M.; Santos, C.A.; Vilela, A.C.F.; Cunha, J.B.M. Chemical, physical, structural and morphological characterization of the electric arc furnace dust. J. Hazard. Mater. 2006, 136, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Kavouras, P.; Ioannidis, T.A.; Kehagias, T.; Tsilika, I.; Chrissafis, K.; Kokkou, S.; Zouboulis, A.; Karakostas, T. EAFD-loaded vitreous and glass–ceramic materials. Eur. Ceram. Soc. 2007, 27, 2317–2323. [Google Scholar] [CrossRef]
- European Waste Catalogue and Hazardous Waste List; Environmental Protection Agency: Wexford, Ireland, 2002.
- EC (European Commission). Council Decision of 19 December 2002 Establishing Criteria and Procedures for the Acceptance of Waste at Landfills Pursuant to Article 16 of and Annex II to Directive 1999/31/EC; EC: Brussels, Belgium, 19 December.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).