Abstract
Bauxite residue (BR), an alkaline industrial waste, is a major byproduct of the alumina production process known as the Bayer process. The Bayer process generates a byproduct known as bauxite residue (red mud). This residue was leached with sulfuric acid in a pilot plant of Mytilineos S.A to recover scandium. Scandium was selectively recovered in pilot-scale experiments using ion exchange. This process generated a raffinate solution containing various dissolved impurities such as aluminum, sodium, calcium, iron, and mainly sulfate ions. The regeneration of the raffinate solution can reduce the cost of the process and minimize the use of H2SO4. The potential of raffinate recycling as a technology for reducing the usage of H2SO4 in the leaching process was evaluated by neutralizing bauxite residue with a raffinate solution before the leaching step. This study aimed to investigate the feasibility of using a raffinate solution for the neutralization of BR, enabling its reuse and improving the process’s environmental sustainability. The neutralization process decreases the pH value of BR pulp with 50% w/v pulp density from 11 to 6. Experimental investigations were carried out to assess the leaching behavior of bauxite residue compared to neutralized bauxite residue (NBR) using sulfuric acid. The obtained results were compared to evaluate the effectiveness of NBR as a substitute for bauxite residue in the leaching process. The consumption of acid during the leaching of neutralized BR was three times less than the BR leaching. An X-ray diffraction (XRD) analysis of BR and NBR was conducted to determine the mineralogical phases of the materials. The results of the study provide valuable insights into potential ways to reduce the cost of the BR leaching process, while also improving its environmental impact by recycling valuable materials.
1. Introduction
Bauxite, the primary source of aluminum, is processed through the Bayer method to extract alumina, resulting in a residual slurry known as red mud. After filter pressing, this slurry becomes a solid residue called bauxite residue (BR). The amount of bauxite residue produced per ton of alumina ranges between 0.8 and 1.5 metric tons [1]. The yearly global production of bauxite residue is estimated to be approximately 120 million tons, and 2.7 billion tons of this material has already been accumulated [2]. The wet bauxite residue slurry has a high pH of approximately 11 and is stored in large tailing ponds, causing significant environmental concerns due to its land occupation and potential for pollution [3]. The effective management and safe disposal of bauxite residue are crucial considerations for the alumina industries, as they directly affect the sustainability of their operations. The valorization of bauxite residue as a secondary raw material and low-cost metal resource offers a potential solution for waste reduction by reintegrating it into the economic cycle [4].
Bauxite residue comprises a range of major metal oxides, such as Fe, Al, Ti, Ca, Si, Na, and minor metal oxides, including V, Ga, REEs/Sc, and other elements [4]. The specific minor metal oxides present in the residue and their concentrations depend on the original chemical composition of the bauxite ore. Additionally, the residue may contain unwashed sodium aluminate solution inclusions [5]. Μajor metal oxides exist in quantities of grams per kilogram, while minor metal oxides are present in quantities of milligrams per kilogram. Mytilineos S.A., a Greek alumina refinery, produces approximately 800,000 tons of BR annually (on a dry basis). Greek BR contains various metals, including a significant amount of scandium (Sc) [6].
One method proposed for recovering scandium (Sc) from BR is through its hydrometallurgical treatment with inorganic acids [7,8]. This technique has been recently developed on a pilot scale [9] and involves the dissolution of major and minor metals with sulfuric acid (H2SO4), generating a pregnant leaching solution (PLS). The dissolved Sc in the PLS was 12.5 ± 0.6 mg/L while Fe and Ti impurities were 219 ± 34.0 and 33.6 ± 3.3 mg/L, respectively. The SO42− content of the PLS was 78 ± 0.8 g/L. After the leaching process, the PLS undergoes an ion exchange process to recover scandium [10]. This process involves passing PLS through an ion exchange column that contains a selective resin. The resin exchanges the scandium cations selectively. The Sc recovery was 83% following the same leaching trend with Fe, while the Si recovery was extremely low. Titanium decreases from 23 to about 0.5 g/L over 4 days of retention time, and iron and aluminum remain in the solution at high concentrations (49.7 and 9.7 g/L, respectively) [11]. Subsequently, the resin is treated with an alkaline solution to elute scandium and other elements. The eluted solution is then subjected to further purification steps, including precipitation, filtration, and other separation techniques, as shown in Figure 1 [11]. As a result of this process, a raffinate solution is generated, which primarily contains H2SO4 and various dissolved impurities, such as aluminum, sodium, calcium, iron, and sulfate ions.
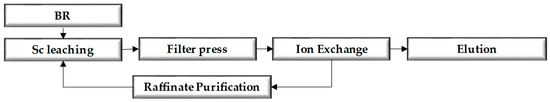
Figure 1.
Flow sheet of the process used by Mytilineos S.A. for the recovery of scandium.
Given the alkaline nature of BR, the consumption of H2SO4 in the BR leaching step comes with a high consumption rate and is the most important cost item of the process. In the case of the Greek BR leaching demonstrated at Mytilineos, the demand for sulfuric acid reached 0.27–0.29 t per t of treated BR, in order to achieve a selective Sc leaching process [9]. To reduce the process’s overall cost, the raffinate solution needs to be reutilized. The technology considered in this study involves neutralizing the BR before leaching with the raffinate solution produced from the ion exchange to reduce acid consumption in the new leaching. This study compares leaching experiments conducted with the conventional procedure (direct BR leaching) with experiments conducted using the neutralized bauxite residue (NBR) in order to analyze the final bauxite residues (BR2) and resulting PLS. The findings of this study will provide valuable insights into potential ways to reduce the cost of the BR leaching process.
2. Materials and Methods
The raffinate solution and the BR investigated in this research were obtained from Mytilineos S.A., Agios Nikolaos, Greece. The BR was collected from the alumina refinery post-dewatering through filter presses and subsequent room temperature drying. It was subjected to further drying at 100 °C for a duration of 24 h. The study used analytical reagent grade sulfuric acid (95–97%) from Sigma-Aldrich, St. Louis, MO, USA.
A chemical analysis of the BR and NBR samples was performed after their complete dissolution by alkali fusion. The alkali fusion was carried out by mixing 0.1 g of BR with 1.5 g of potassium nitrate and 1.5 g of lithium tetraborate, followed by heating the mixture in a platinum crucible at 1000 °C for 1h. The generated solutions were measured by ICP-OES (Optima 7000, PerkinElmer, Akron, OH, USA) analysis and atomic absorption spectroscopy AAS (PinAAcle 900T, PerkinElmer, Akron, OH, USA). The total sulfate ion content was determined by a gravimetric method of BaSO4 formation. Free H2SO4 acid content was determined by direct titration with NaOH standard solution at pH 3.5.
The X-ray diffraction patterns were collected using a MiniFlex 600 benchtop diffractometer (Rigaku, Tokyo, Japan), equipped with a D/tex ultra detector. The diffractometer operated at 40 kV and 15 mA (600 W) with Cu-Kα radiation. Diffraction data were collected over a 2θ range from 5 to 90°, in 0.02° steps, and at a rate of 5° per minute. The characterization of the examined materials was performed in DIFFRAC. EVA software V5.1 using the ICDD databases PDF-4+ 2022 and PDF-4 Minerals 2022 [12].
The pH measurements were carried out with a pH meter Metrohm 913 laboratory version equipped with a Porotrode 3m KCl pH electrode.
For the neutralization experiments, a 50% w/v pulp density of BR pulp with deionized water was prepared (168.8 g of BR with 337.6 mL of water). The pulp was introduced into a beaker and subjected to continuous stirring facilitated by a magnetic stirrer. The pH was monitored at 5 min intervals using a pH meter, while the addition of the raffinate solution was carefully added in a gradual manner employing a titration burette.
The leaching experiments of the residues were carried out using a 500 mL round-bottom glass reactor placed in a thermostatically controlled mantle at 300 rpm and 80 °C. The reactor was equipped with a four-necked lid, a thermocouple was fitted to one of the openings to constantly control the temperature, a porotrode electrode for the systematic measurement of pH, and a condenser was employed to facilitate vapor cooling. Leaching experiments were repeated twice to assess reproducibility. The resulting leachates were analyzed by ICP-OES and AAS to determine the Fe, Al, Si, Na, Ca, Mg, Ti, Cr, Zr, Ni, Zn, V, Ga, and Sc content in the solutions.
3. Results and Discussion
3.1. Characterization of the Bauxite Residue and Raffinate Solution
Table 1 presents the chemical composition of the BR sample. Iron(III) oxide is the major oxide in the BR followed by alumina, silica, calcium oxide, sodium oxide and titanium oxide. Other oxides are also present in BR at low concentrations (e.g., oxides of magnesium, chromium, zirconium, nickel, vanadium, gallium, zinc, and scandium).

Table 1.
Chemical composition of the BR after the complete dissolution of the BR via alkali fusion.
Figure 2 illustrates the mineralogical analysis of the BR sample. According to our findings, hematite (Fe2O3), diaspore (AlO(OH)), cancrinite (Na8(Si6Al6O24)(H0.88(CO3)1.44)(H2O)2), and katoite (Ca3Al3.5O4.5(OH)7.5 are the major constitutes of the BR, while Al-goethite (Fe0.93Al0.07O(OH), gibbsite (Al(OH)3), calcite (CaCO3), perovskite (CaTiO3), chamosite ((Mg5.036Fe4.964)Al2.724((Si5.7Al2.3)O20(OH)16), and boehmite (AlO1.06(OH)0.94) are present in much lower quantities. Anatase (TiO2) and quartz (SiO2) are also present in trace amounts. The chemical analysis of the raffinate solution used in this study can be seen in Table 2. The solution is mostly composed of sulfate ions (SO42−), 24% of them are free sulfuric acid, sodium (Na) ions, and aluminum (Al) ions.
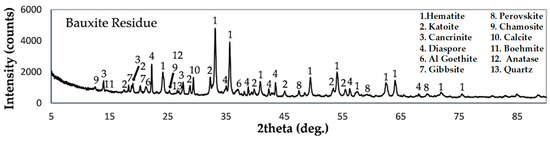
Figure 2.
XRD pattern of non-neutralized bauxite residue.

Table 2.
Chemical composition of raffinate solution.
3.2. Neutralization of BR
The impact of neutralizing the BR was investigated through a sequential experimental approach, as illustrated in Figure 3. The study involved two distinct processes: (1) neutralization of the bauxite residue slurry using a raffinate solution derived from bauxite residue, and (2) subsequent acidic leaching of both the non-neutralized (BR) and neutralized bauxite residue (NBR). This experimental design aimed to assess the effects of neutralization on the overall leaching process, providing valuable insights into the potential benefits of neutralization in the context of BR treatment.
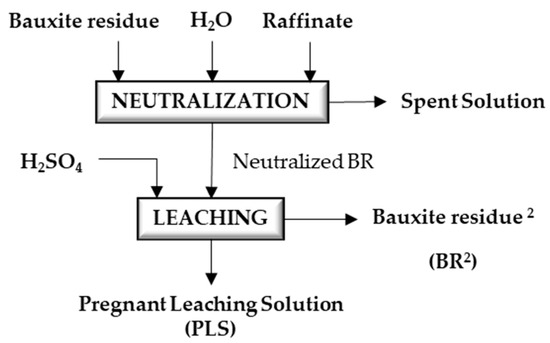
Figure 3.
Flow sheet for the neutralization of bauxite residue, followed by metal recovery from neutralized bauxite residue via leaching with sulfuric acid.
For the neutralization process, the BR pulp was added to a beaker through constant magnetic stirring. The raffinate solution was then added gradually until the pH of the solution was close to 6. The pH of the BR pulp was equal to 10.8 ± 0.16, while the pH of the neutralized BR pulp was equal to 7.03 ± 0.15. The chemical analysis of the NBR sample is reported in Table 3 and the chemical analysis of the resulting spent solution can be seen in Table 4. The XRD graph of the BR and the NBR is shown in Figure 4a,b, respectively.

Table 3.
Chemical composition of the NBR after complete dissolution of the NBR by alkali fusion.

Table 4.
Chemical composition of spent solution after neutralization process and of raffinate solution.
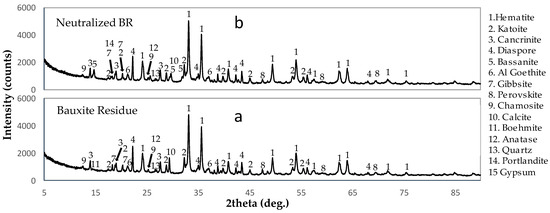
Figure 4.
XRD patterns of the examined materials (a) BR, (b) neutralized BR.
The chemical analysis of the NBR sample is reported in Table 3. The observed concentrations closely align with the chemical analysis results of the BR sample (Table 1), exhibiting minor variations.
Concerning the sulfate content of the solution, approximately 86% of the total sulfate ions (SO42−) were consumed from the raffinate solution to neutralize the BR, effectively utilizing all the free H2SO4 acid [11]. The chemical analysis results of the spent solution revealed that various elements, including Fe, Al, Ti, Cr, Zr, Ni, Zn, V, Ga, and Sc, were precipitated from the raffinate solution. Conversely, the concentration of Mg in the resulting spent solution exhibited a significant increase of up to 470% (67.1 mg/L), while the Ca concentration rose by 13% (79.7 mg/L). The elevated concentration of Mg in the spent solution can be justified by the partial dissolution of the chamosite mineral present in the BR [13], which is also subtly evident in the XRD graphs. In terms of Ca, the XRD graphs indicate the significant dissolution of calcite upon its reaction with H2SO4, resulting in the formation of bassanite (Ca2(SO4)2(H2O)). Additionally, a portion of the calcium is transferred to the aqueous phase, while a small portion forms portlandite (Ca(OH)2), due to kinetic factors such as rapid filtration and no aging period. Regarding Si and Na, approximately 85% of Si and 60% of Na were precipitated from the raffinate solution.
3.3. Leaching Experiments
For the leaching process, in accordance with the procedure developed in the pilot scale [11] a 42.2% p.d. of BR pulp (168.8 g of BR mixed with 400 mL of deionized water) was introduced into the reactor with constant stirring for 1 h. The H2SO4 (18 M) was added gradually until the pH of the pulp reached a pH value close to 2.3. Experiments were performed in duplicates with a 1h duration. The chemical analysis results are presented in Table 5.

Table 5.
Chemical analysis of PLS resulting from the leaching processes of BR and NBR.
In the leaching process involving the non-neutralized BR, the BR pulp was formed in the reactor with a starting pH close to 11. To achieve the target pH value of 2.3, 30 mL of concentrated H2SO4 was required. The effect of acid addition on the pH of the solution for both BR and NBR is presented in Figure 5. The chemical analysis of the obtained PLS can be seen in Table 5. The XRD graphs of the residues after the non-neutralized BR leaching and the NBR leaching are presented in Figure 6a,b, respectively.
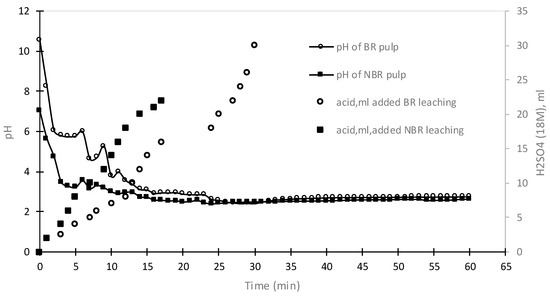
Figure 5.
The effect of acid addition (H2SO4) on the pH of the non-neutralized and neutralized bauxite residue samples.
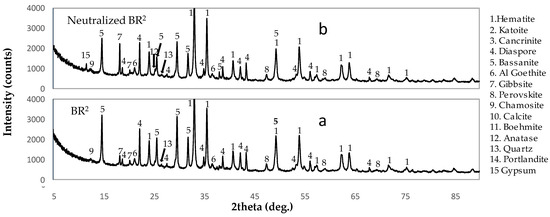
Figure 6.
XRD patterns of the examined materials (a) BR2, and (b) neutralized BR2.4. Conclusions.
In the leaching process involving NBR, the NBR pulp was formed with a pH close to 7, and 23 mL of 18 M H2SO4 was added for the leaching process. The amount of Sc in the PLS was equal to 13.4 mg/L (22.4% recovery). The leaching process with the NBR demonstrated a reduced acid consumption of 23.3% compared to the leaching process with the non-neutralized BR. This indicates that the neutralization of BR prior to leaching effectively reduced the amount of sulfuric acid required for the process. However, it is worth noting that the NBR leaching resulted in a lower recovery of scandium, with an overall recovery of 22.4% compared to 25.8% in the non-neutralized BR leaching.
Significantly higher aluminum levels were observed in the PLS obtained from the NBR leaching. This can be attributed to the precipitation of 8.9 g/L of aluminum from the raffinate solution during the neutralization process that was redissolved during leaching. A comparison of the XRD graphs of the NBR2 and BR2 reveals less formation of bassanite in the case of NBR leaching. This can probably be explained since the leaching process of the NBR is less aggressive compared to the non-neutralized BR leaching, as it starts from an almost neutral pH.
In this study, our investigation focused on the potential of utilizing a raffinate solution, obtained from the leaching process of BR, developed on the pilot plant of MYTILINEOS. The aim was to assess the feasibility of recycling the raffinate solution to neutralize the BR prior to leaching, with the objective of reducing the operating costs.
Concerning the neutralization step, our findings demonstrated that the effectiveness of the neutralization process in reducing the pH of the BR pulp from an initial value of 10.8 ± 0.16 to 7.03 ± 0.15 using a 50% w/v p.d. of BR pulp, making it easier to handle. The chemical analysis results of the spent solution revealed that various elements, including Fe, Al, Ti, Cr, Zr, Ni, Zn, V, Ga, and Sc, were precipitated from the raffinate solution, leaving behind only Na, Ca, Mg, Si, and some sulfate ions. Notably, approximately 86% of the total SO42− content and all the free acids were consumed during this process.
The leaching experiments conducted with the non-neutralized BR and neutralized BR with sulfuric acid revealed that the acid consumption during the leaching of NBR was three times lower compared to the non-neutralized BR leaching. The recovery rates of scandium from the PLS in the BR and NBR leaching processes were determined to be 25.8% and 22.4%, respectively.
Overall, the results of this study highlight the potential benefits of utilizing the raffinate solution produced after the ion exchange process, through recycling it for the neutralization of the BR. This approach can contribute to a reduction in cost and the minimization of environmental impacts associated with the process, since the resulting spent solution has lower impurities. These findings provide valuable insights towards the sustainable management of bauxite residue and the recovery of valuable elements, such as scandium, from industrial waste streams.
Author Contributions
Conceptualization, A.T., E.B., D.M. and D.P.; methodology, A.T., E.B. and D.M.; validation, P.D., D.M., E.B. and D.P.; data curation A.T., D.K. and M.P.; writing—original draft preparation, A.T., D.K., M.P. and D.M.; writing—review and editing, D.M., E.B. and D.P.; supervision E.B. and D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by EIT, SCALE-UP KIC Project (2022–2024), project number 21013.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Balomenos, E.; Panias, D.; Paspaliaris, I. Energy and Exergy Analysis of the Primary Aluminum Production Processes: A Review on Current and Future Sustainability. Miner. Process. Extr. Metall. Rev. 2011, 32, 69–89. [Google Scholar] [CrossRef]
- Klauber, C.; Gräfe, M.; Power, G. Bauxite Residue Issues: II. Options for Residue Utilization. Hydrometallurgy 2011, 108, 11–32. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.X.; Liu, X.M. Recovery of Scandium from Bauxite Residue—Red Mud: A Review. Rare Metals 2016, 35, 887–900. [Google Scholar] [CrossRef]
- Barry, T.S.; Uysal, T.; Birinci, M.; Erdemoğlu, M. Thermal and Mechanical Activation in Acid Leaching Processes of Non-Bauxite Ores Available for Alumina Production—A Review. Min. Metall. Explor. 2019, 36, 557–569. [Google Scholar] [CrossRef]
- Angelopoulos, P.; Georgiou, M.; Oustadakis, P.; Taxiarchou, M.; Karadağ, H.; Eker, Y.; Dobra, G.; Boiangiu, A.; Demir, G.; Arslan, S.; et al. Preliminary Characterization of Three Metallurgical Bauxite Residue Samples. Mater. Proc. 2021, 5, 66. [Google Scholar]
- Ochsenkuehn-Petropoulou, M.; Tsakanika, L.A.; Lymperopoulou, T.; Ochsenkuehn, K.M.; Hatzilyberis, K.; Georgiou, P.; Stergiopoulos, C.; Serifi, O.; Tsopelas, F. Efficiency of Sulfuric Acid on Selective Scandium Leachability from Bauxite Residue. Metals 2018, 8, 915. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H. Metallurgical Process for Valuable Elements Recovery from Red Mud—A Review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Metallurgical Processes for Scandium Recovery from Various Resources: A Review. Hydrometallurgy 2011, 108, 100–108. [Google Scholar] [CrossRef]
- Balomenos, E.; Davris, P.; Apostolopoulou, A.; Marinos, D.; Mikeli, E.; Toli, A.; Kotsanis, D.; Paschalis, G.; Panias, D. Investigations into Optimized Industrial Pilot Scale BR Leaching for Sc Extraction. In MS Annual Meeting & Exhibition; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Davris, P.; Balomenos, E.; Nazari, G.; Abrenica, G.; Patkar, S.; Xu, W.-Q.; Karnachoritis, Y. Viable Scandium Extraction from Bauxite Residue at Pilot Scale. Mater. Proc. 2022, 5, 129. [Google Scholar]
- Balomenos, E.; Nazari, G.; Davris, P.; Abrenica, G.; Pilihou, A.; Mikeli, E.; Panias, D.; Patkar, S.; Xu, W.Q. Scandium Extraction from Bauxite Residue Using Sulfuric Acid and a Composite Extractant-Enhanced Ion-Exchange Polymer Resin. In Minerals, Metals and Materials Series; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2021; pp. 217–228. [Google Scholar]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Gentzmann, M.C.; Paul, A.; Serrano, J.; Adam, C. Understanding Scandium Leaching from Bauxite Residues of Different Geological Backgrounds Using Statistical Design of Experiments. J. Geochem. Explor. 2022, 240, 107041. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).