1. Introduction
Rare-earth elements (REEs), also known as rare-earth metals, are a group of 17 naturally occurring elements in the periodic table that includes yttrium and scandium as well as lanthanides [
1]. The importance of REEs is growing in advanced technologies and renewable energy applications. REEs appear as the key components employed in numerous applications in various modern technological, environmental, and economic domains due to their catalytic, metallurgical, nuclear, electrical, magnetic, and luminescent properties. As a result, demand for REEs has increased significantly in recent years [
2]. Technology advancements boosted the demand for REEs, and their limited global distribution has increased REE prices [
3]. Therefore, recycling and the utilization of secondary rare-earth resources for a sustainable and circular economy appear to be an alternative to meet the demand for these raw materials in the future [
4,
5]. Fluorescent lamps, tube lamps, cathode ray tubes, LEDs, hard disks, wind turbines, electric motors, and end-of-life batteries are examples of secondary REE sources [
6].
A significant amount of REEs are used in the preparation of luminescent materials, commonly known as phosphors. Fluorescent lamps contain a significant amount of one or more rare-earth phosphors: A typical 40 W fluorescent lamp contains 4 to 6 g or 2% by mass of phosphor powder, depending on the type and location of manufacturing [
7]. These compositions might appear insignificant at the outset; however, to produce the same quantity of REEs, 19 tons of ore minerals must be processed for 1 ton of phosphorous powder. Phosphorus powder can also be a cleaner alternative source due to its lower elemental composition—apart from REEs—when considering the mineral content [
6].
High-performance fluorescent lamps contain a combination of red, green, and blue rare-earth phosphors. The powder coating of a typical tri-phosphor fluorescent lamp contains roughly 55% red (YOX), 35% green (LAP, CAT), and 10% blue (BAM) phosphors. While red phosphorus mainly consists of yttrium (Y) and europium (Eu), approximately 10% of green phosphorus and less than 5% of blue phosphorus constitute terbium (Tb) [
8]. The contents of YOX, CAT, LAP, and BAM constructs are represented in
Table 1.
Although various methods are used for the recovery of these elements, selective separation of REEs is extremely challenging due to small variation in ionic radius, propensity to interact with hard-sphere-based donor atoms and predominance of +3 oxidation state within the lanthanide series. For the selective leaching of REEs and their specific separation by eliminating impurities, different approaches such as solvent extraction (SX) [
10,
11], precipitation [
12], supercritical fluid extraction [
13], ion exchange [
14], and ionic liquid methods [
10,
15] have been developed. Due to its highly efficient extraction capability and selectivity, SX is one of the most prevalent hydrometallurgical processes. Solvent extraction has also been developed industrially to separate REEs independently since this method can treat larger volumes of diluted charged solutions at an industrial scale [
16].
In this work, the effects of certain parameters on the leaching process, which is the initial step of hydrometallurgical methods, such as the type and concentration of extractant, the reaction temperature and time, and the solid/liquid ratio, were investigated. SX parameters were optimized by determining the solvent type, solvent concentration, organic/aqueous phase ratio, mixing and resting time, pH effect, number of stages, type, and concentration of stripping solution in the next part of the study.
2. Materials and Methods
Fluorescent powders were supplied from Exitcom Recycling Company, Kocaeli, Turkiy the project partners. HCl, H2SO4, and oxalic acid were procured from Merck, Germany (provided by the distributor in Türkiye, Labor, Istanbul, Türkiye). Cyanex 923, Cyanex 572, and D2EHPA were obtained from Solvay S.A. Kerosene of technical grade was used. All chemicals were of reagent grade and used without additional purification. Ultrapure water from the Stakpure system was used in the experiments. Magnetic stirrers (Heidolph, provided by the distributor in Türkiye, Info Endustri, Istanbul, Türkiye) were used for mixing and shaking processes. For measuring pH, a pH meter (Mettler Toledo, ABD, provided by Süber Glass Laboratory Products and Chemicals, Ankara Türkiye) was utilized. All of the experiments conducted were carried out at atmospheric conditions. End-of-life fluorescent lamps were pulverized after crushing, grinding, and mercury removal process. The supplied end-of-life fluorescent powder was analyzed by XRF method for determination of composition prior to any processing.
3. Result and Discussion
Table 2 represents the REEs composition of the powder with approximately 8% of the total weight.
The phase structure of the powder was determined by XRD analysis (
Figure 1), and XRF analysis confirmed the composition of the given powder. The primary phase was identified as Y
2O
3: Eu
2+ and the structure also contained (Ce,Tb)MgAl
11O
19, BaMgAl
10O
17:Eu
2+, and LaPO
4:Ce
3+,Tb
3+. Then, the chemical recovery process for fluorescent powders containing high REEs was initiated.
After the composition analyses of the end-of-life fluorescent lamp powders via XRD analysis method, the hydrometallurgical method was preferred for the recovery of REEs. At the initial step, leaching experiments were carried out at different S/L ratios (1/5, 1/10, and 1/20 in 5 M HCl acid at room temperature). Gelation was observed using a 1/5 solid/liquid ratio; thus, leaching could not succeed. Although the highest efficiency was obtained at a 1/20 S/L ratio, the industrial feasibility of the process was low. A 1/10 solid/liquid ratio was accepted as an optimum parameter for the S/L ratio.
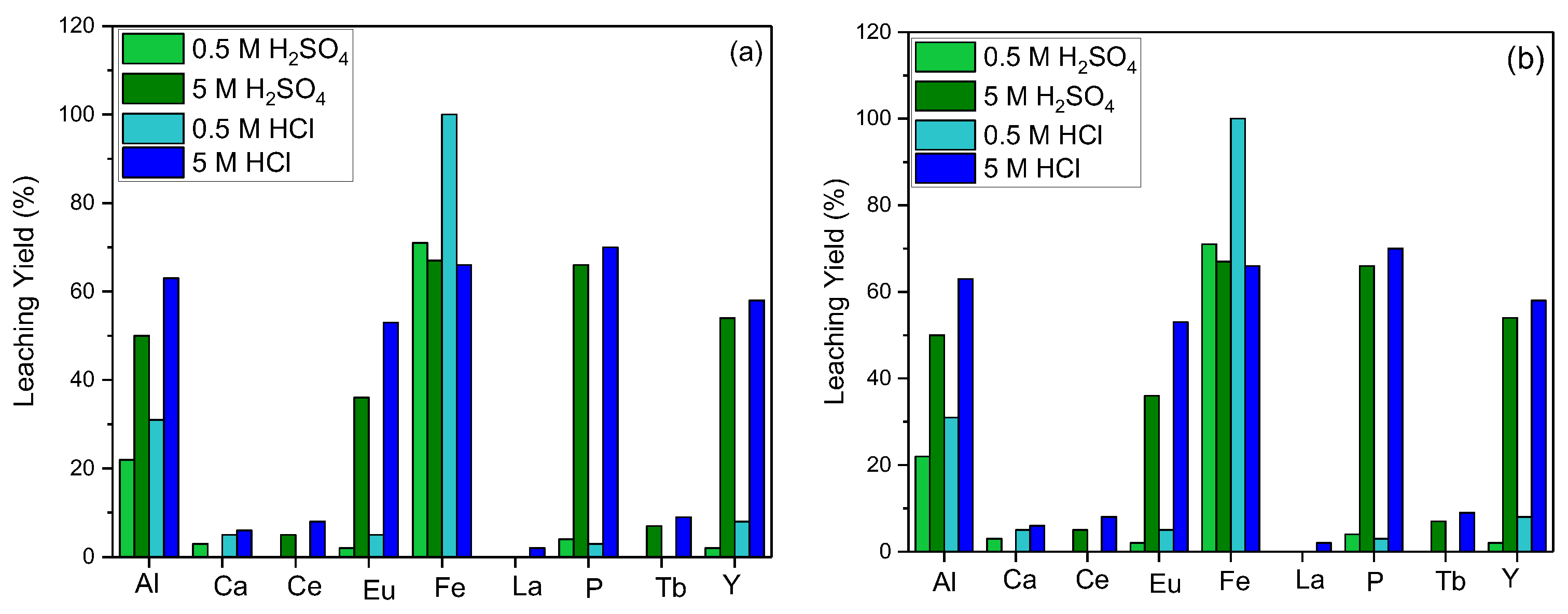
In order to maximize the leaching process efficiency, the effect of acid molarity for various acid types was investigated. For the hydrometallurgical process, hydrochloric (HCl) and sulfuric (H
2SO
4) acids were used preferably, and the leaching efficiency at different molarities of the acids was studied. The efficiency of REE leaching was found to be directly proportional to the acid concentration for both types of acids and contaminants in the structure, such as Al, Fe, and P dissolving into the leaching solution rapidly (
Figure 2).
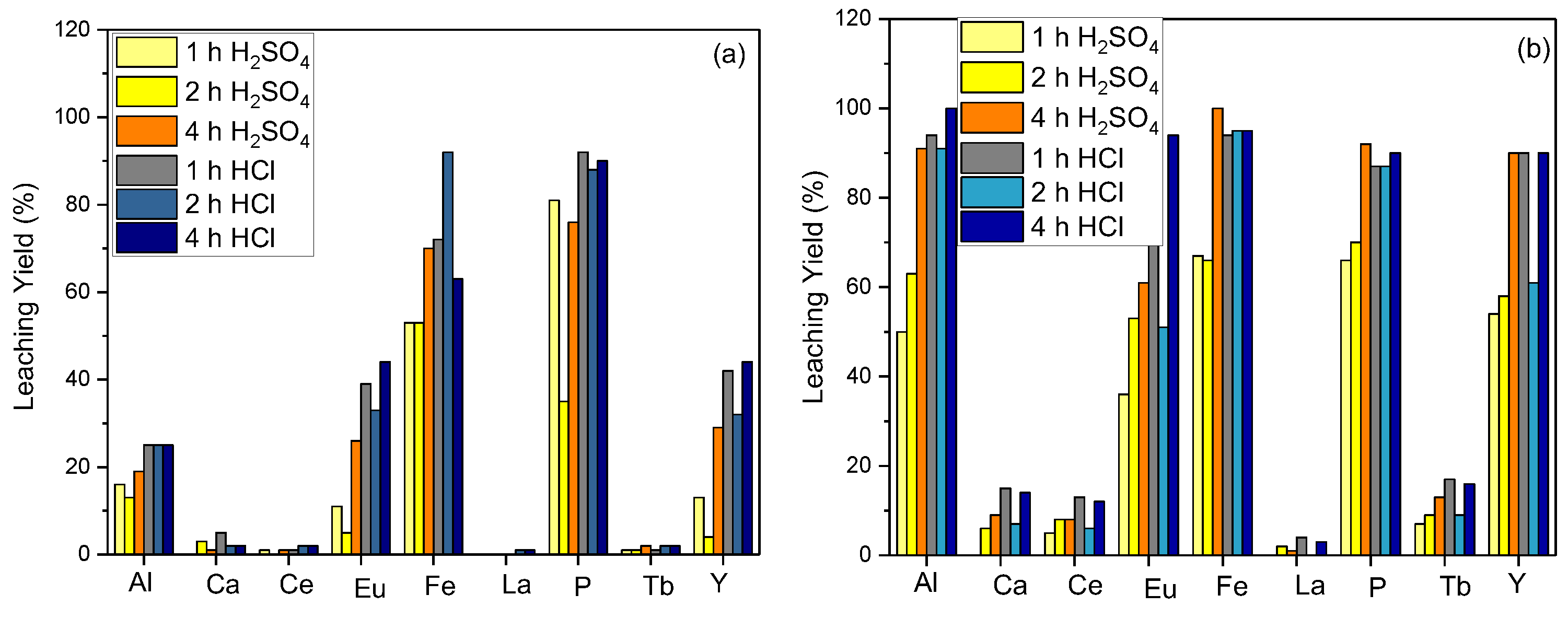
The effect of the reaction time on the leaching efficiency was evaluated for the leaching parameter optimizations. The optimal yield was obtained when the material was leached in 5 M HCl acid for 4 h, as both reaction time and temperature increased.
Figure 3a represents the leaching efficiency for Y for 2 and 4 h of reaction time at room temperature. The efficiency increases to 90% as the reaction temperature is raised to 80 °C (
Figure 3b) For leaching efficiency capacities of 2 h and 4 h, approximations of the values were obtained as 95% and 90%, respectively. According to the obtained efficiency for the 4 h reaction time, a 5% yield loss was disregarded since it is economically feasible in commercial applications, and it was determined that the next investigations would leach for 2 h at 80 °C.
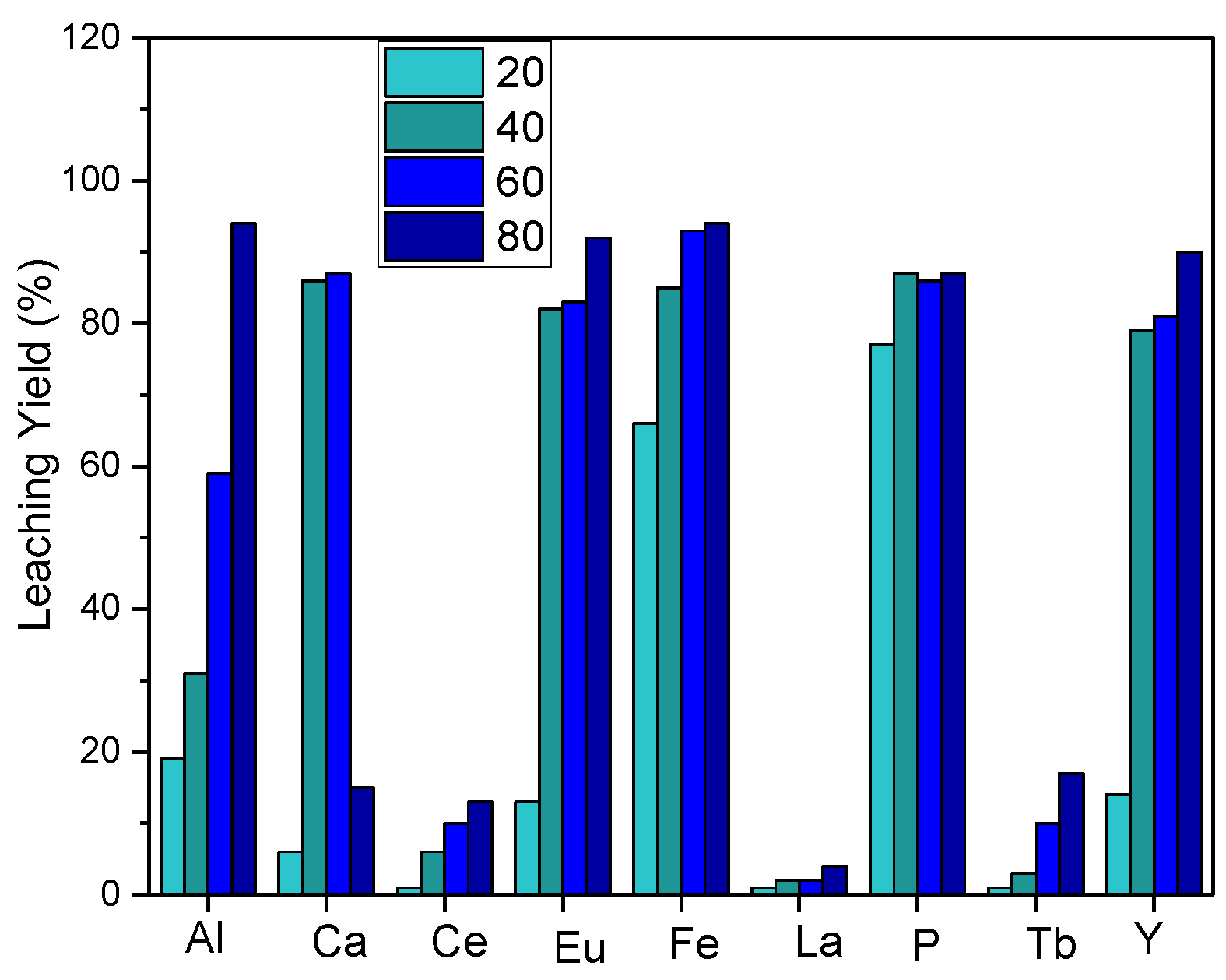
For further evaluation of the temperature effect on leaching, experiments were carried out by reducing the temperature range, and the results are depicted in
Figure 4. Leaching efficiency increased as the leaching temperature increased, and red phosphorus was effectively transported to the leaching solution.
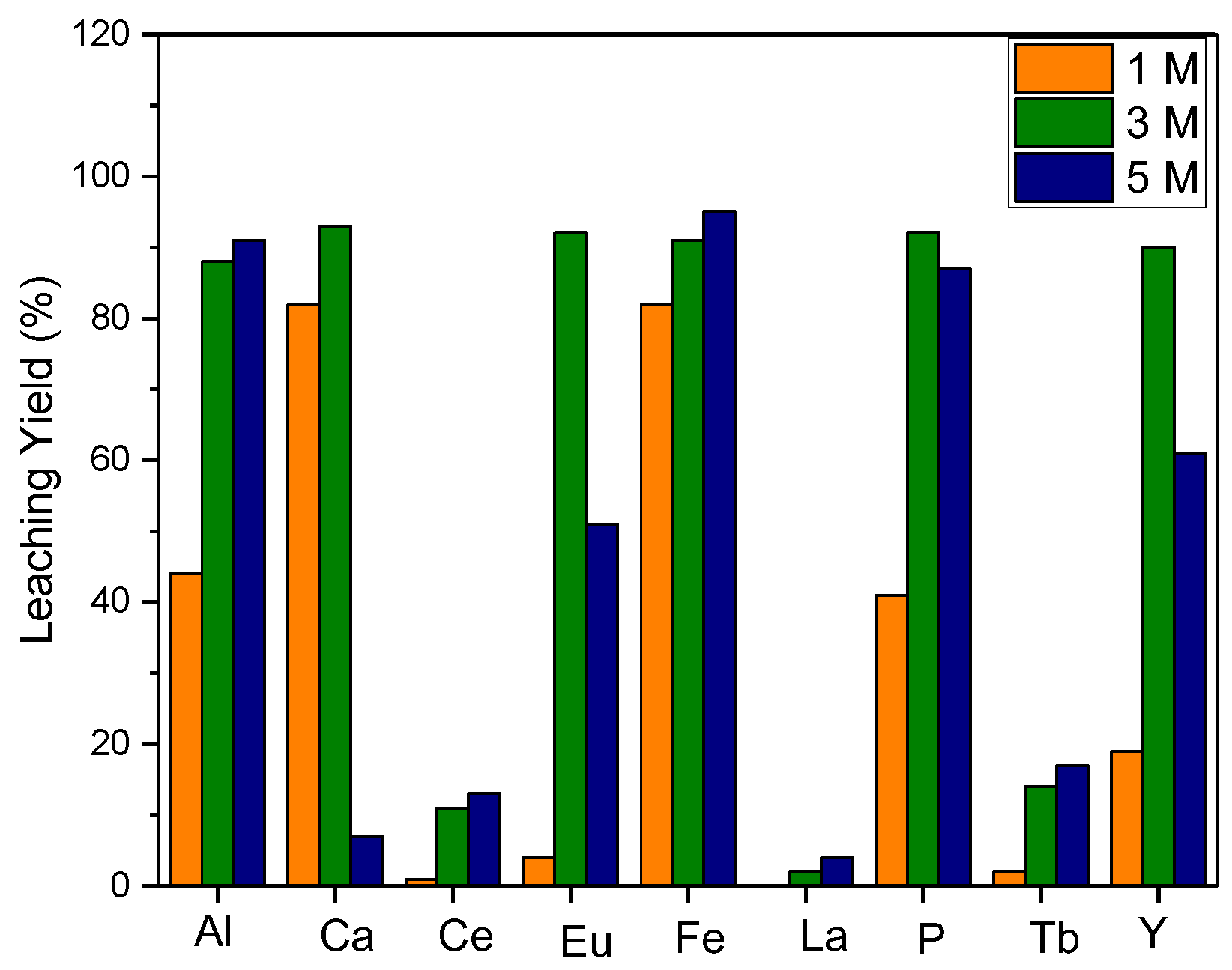
The leaching process in a 5 M HCl solution for 2 h resulted in 90% efficiency. In order to avoid highly concentrated acid consumption, optimization for acid concentration was performed: various HCl solution concentrations were investigated for a 2 h leaching process. Leaching efficiency in solutions of 3 M and 5 M HCl solutions were found to be relatively high, and it was determined that a 3 M HCl solution is appropriate for the amount of acid utilized (
Figure 5).
The leachability of various phosphor types obtained from end-of-life fluorescent lamp powder are different from one another, but red phosphors’ YOX structure has a higher dissolution efficiency. Phosphorus dissolves as a halo (Ca
10(PO
4)
6FCl: Sb
3+, Mn
2+) > YOX (Y
2O
3:Eu
3+) > LAP (LaPO
4:Tb
3+, Ce
3+) according to the literature [
6]. The difference in the dissolving rate was attributed to YOX crystal lattice’s lower level of complexity as compared to the LAP and CAT structures.
Extraction of red phosphor from three phosphor mixtures was carried out at room temperature, and Cyanex 923, Cyanex572, and D2EHPA were used in the initial studies to investigate the extractant effect utilized in the subsequent series of tests. The organic phase was composed of 35%, 33%, and 20% v/v extractant in kerosene, and the O/A ratio was 2/1, 2/1, and 1/1, respectively. Sodium hydroxide solution was added to adjust the pH values of the leaching liquors, and after achieving the required pH level, the organic and aqueous phases remained in contact until equilibrium.
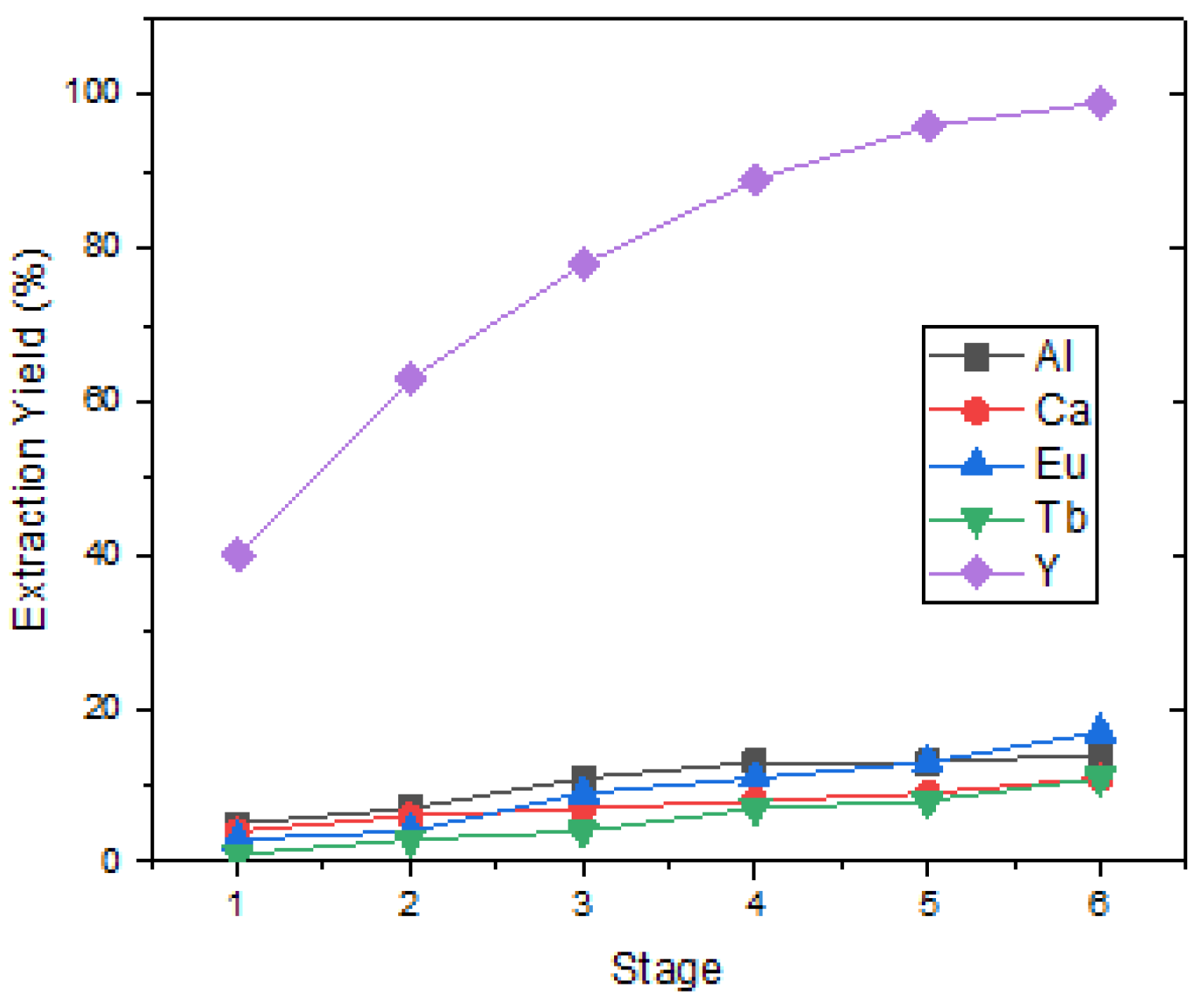
Y was dissolved in the first-stage leach solution at a high concentration, and pregnant leaching solutions also contain valuable elements such as Eu, but also Ca, Al, Si, P, and Mn impurities in small amounts. DEHPA has been demonstrated to be the most effective organic solvent for separating Y from Eu and impurities. Rare-earth metals in the feed solution were quantitatively transferred to the organic phase with 20%
v/
v D2EHPA in kerosene at pH 0.5. In these conditions, the separation took six stages of load and stripping. The six-stage Y loading efficiency was calculated as approximately 98% and the stripping efficiency as 97%. The purity of Y
2O
3 in the precipitation after the stripping process was analyzed as 76.94%. In addition to element Y, there were also elements Eu, Ca, Al, and Tb as impurities.
Figure 6 shows that Y was loaded at 99% when the six-stage loading process was applied.
4. Conclusions
The recovery of rare-earth metals from acidic leaching solution of phosphor powders in end-of-life fluorescent lamps was investigated using a liquid–liquid extraction technique. Initially, acid leaching was used to obtain rare-earth elements from end-of-life phosphor powder, and it was found that Y and Eu are dissolved in acidic solution relatively easily, whereas a significant amount of energy is required for the leaching of other rare-earth elements. Although it is challenging to separate Y, Eu, La, and Ce from metal impurities such as Fe, Al, and Zn, this study delved into the extraction of rare earths in preference to such metal impurities. Cyanex 923, Cyanex 572, and D2EHPA, all of which are kerosene-based extractants with a strong affinity for rare-earth metal ions, were employed as the extraction solvents. Among them, D2EHPA demonstrated the highest efficiency. Optimal extraction efficiency was achieved using 20% v/v D2EHPA in kerosene, maintaining an O/A (organic/aqueous) ratio of 1/1 at a pH level of 0.5.