Characterization of Waste from the Dicalcium Phosphate Industry as a Potential Secondary Source of Rare Earth Elements †
Abstract
:1. Introduction
2. Case Studies
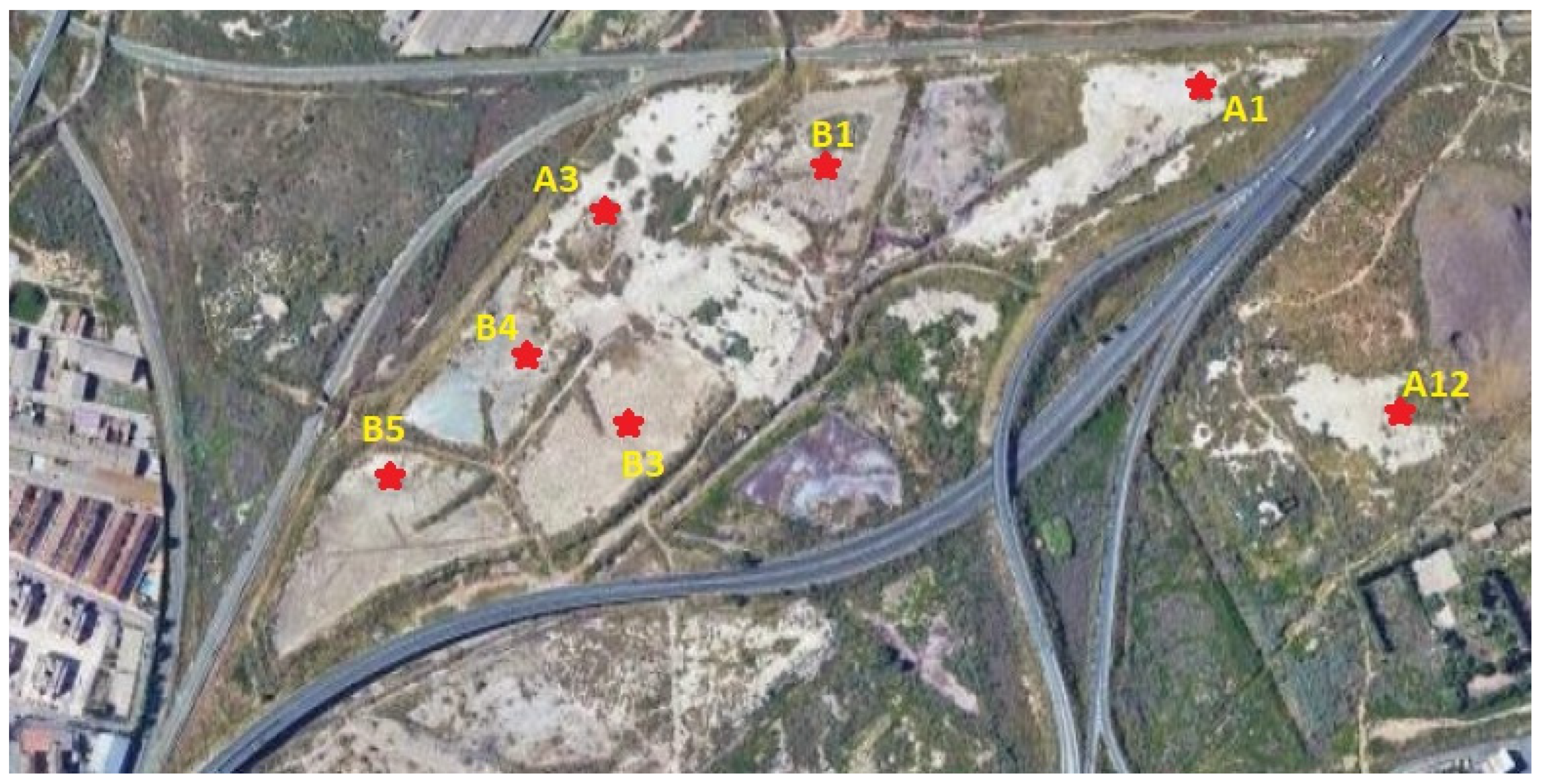
2.1. El Hondón-Cartagena
2.2. Flix
3. Materials and Methods
3.1. Sampling
3.2. Sample Analysis
4. Results and Discussion
4.1. ICP and ISE
4.1.1. Major Elements
4.1.2. Trace Elements, including REEs
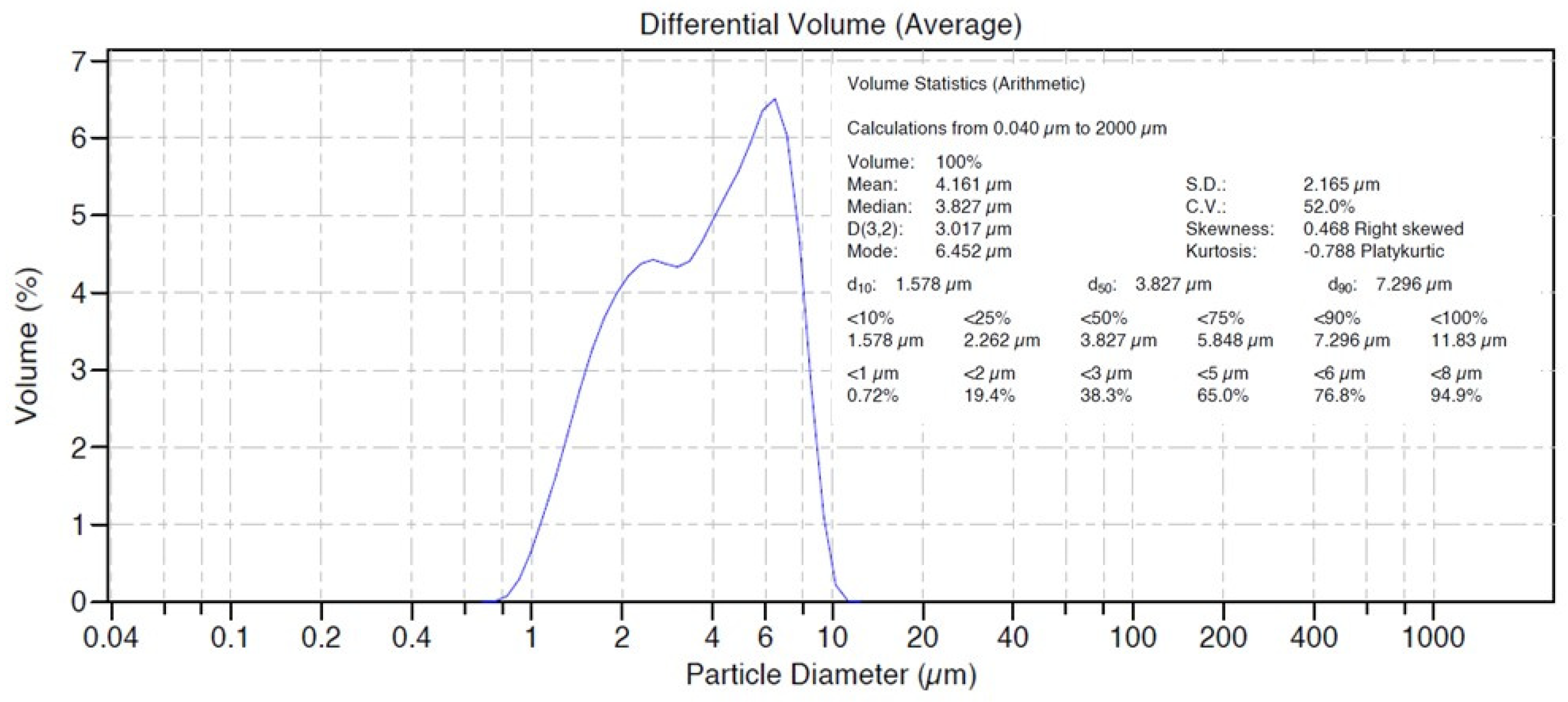
4.2. Particle Size Analysis
4.3. XRD
4.4. SEM and TEM
4.5. µXRF
5. Economic Assessment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arruda-Neto, J.D.T.; Tavares, M.V.; Filadelfo, M. Concentrations of uranium in animal feed supplements: Measurements and dose estimates. J. Radioanal. Nucl. Chem. 1997, 221, 97–104. [Google Scholar] [CrossRef]
- Mola, M.; Palomo, M.; Peñalver, A.; Aguilar, C.; Borrull, F. Distribution of naturally occurring radioactive materials in sediments from the Ebro river reservoir in Flix (Southern Catalonia, Spain). J. Hazard. Mater. 2011, 198, 57–64. [Google Scholar] [CrossRef]
- Cánovas, C.R.; Macías, F.; Pérez-López, R.; Nieto, J.M. Mobility of rare earth elements, yttrium and scandium from a phosphogypsum stack: Environmental and economic implications. Sci. Total Environ. 2018, 618, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Casacuberta, N.; Masqué, P.; García-Orellana, J. Fluxes of 238U decay series radionuclides in a dicalcium phosphate industrial plant. J. Hazard. Mater. 2011, 190, 245–252. [Google Scholar] [CrossRef] [PubMed]
- García-Talavera, M.; Matarranz, J.L.M.; Salas, R.; Ramos, L. A regulatory perspective on the radiological impact of NORM industries: The case of the Spanish phosphate industry. J. Environ. Radioact. 2011, 102, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Solé, V.; Papillon, E.; Cotte, M.; Walter, P.; Susini, J. A Multiplatform Code for the Analysis of Energy-Dispersive X-ray Fluorescence Spectra. Spectrochim. Acta Part B At. Spectrosc. 2006, 62, 63–68. [Google Scholar] [CrossRef]
- Statista. Fluorspar Price in the United States from 2014 to 2022. Available online: https://www.statista.com/statistics/1051742/fluorspar-price-us/ (accessed on 27 June 2023).
- Statista. Average Price of Selected Rare Earth Oxides from 2015 to 2022. Available online: https://www.statista.com/statistics/617249/price-range-of-selected-rare-earth-oxides/ (accessed on 27 June 2023).
- USGS. Fluorspar in the Fourth Quarter 2022. Mineral Industry Surveys; USGS: RestoN, VA, USA, 2022. [Google Scholar]
- Institut für Seltene Erden und Strategische Metalle AG. Metal Prices. 2023. Available online: https://institut-seltene-erden.de/ (accessed on 13 June 2023).


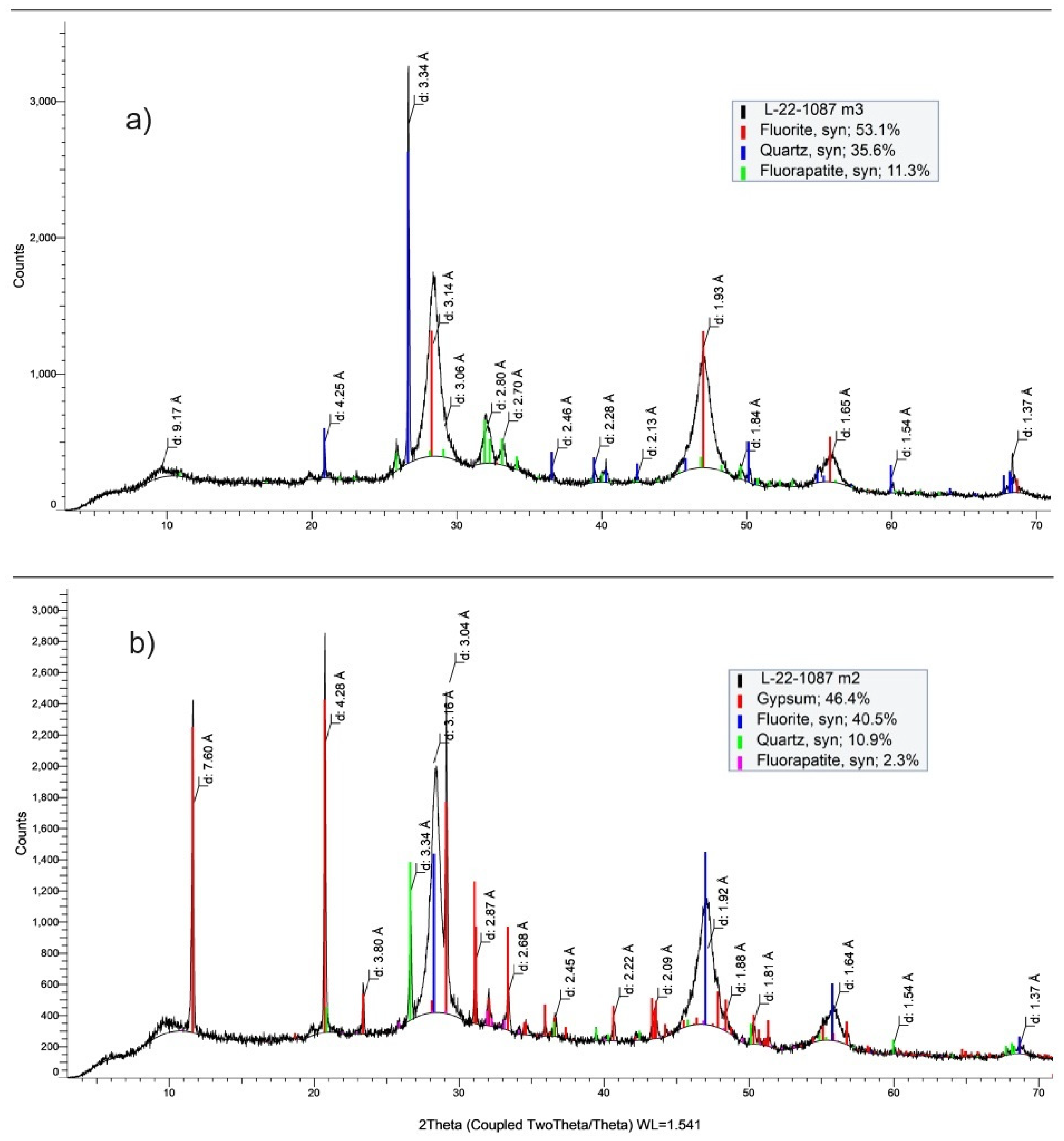
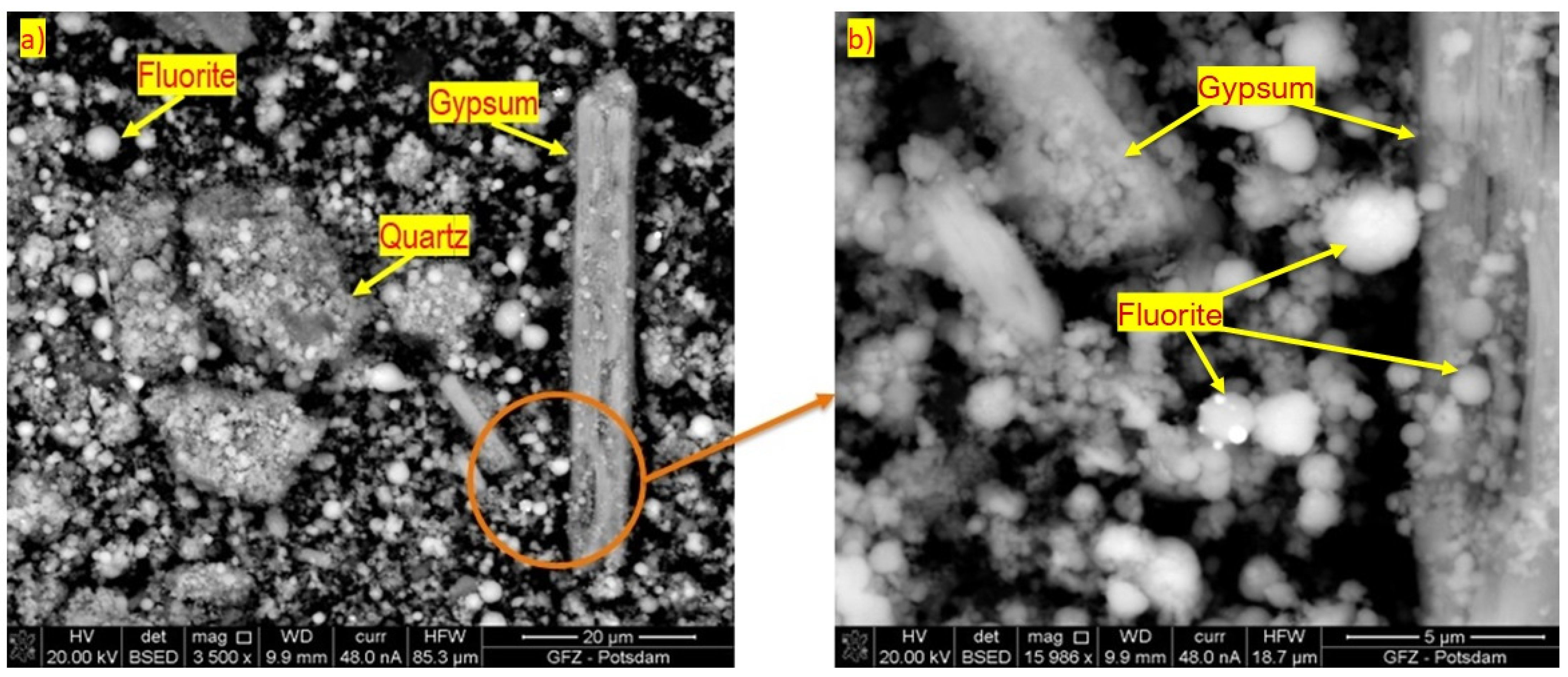

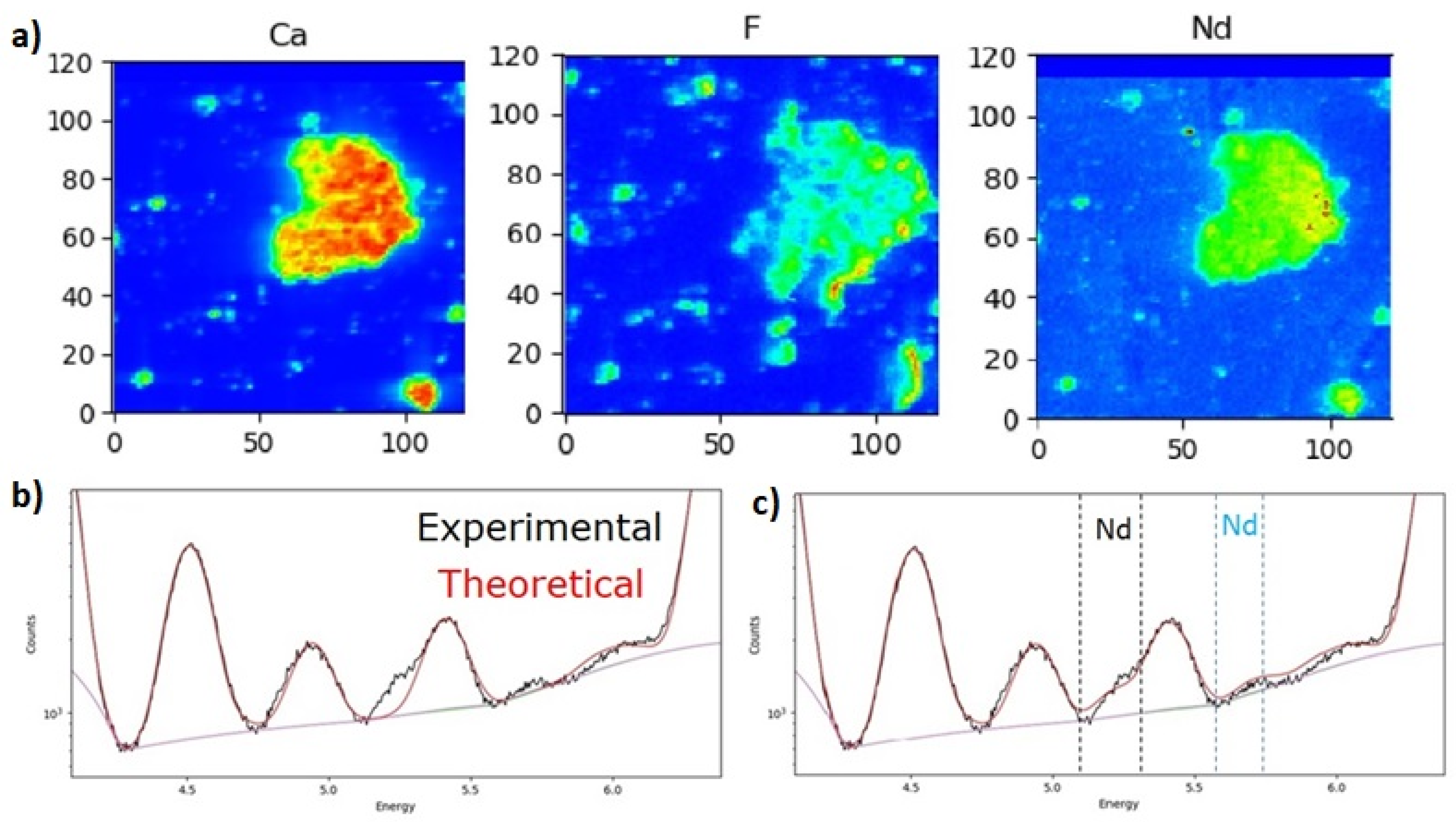
| Composition | FL | EH |
|---|---|---|
| F | 18.70 | 16.70 |
| SiO2 | 12.02 | 7.49 |
| Al2O3 | 1.38 | 2.20 |
| Fe2O3(T) | 0.78 | 1.13 |
| MnO | 0.01 | b.d.l. |
| MgO | 0.30 | 0.41 |
| CaO | 42.52 | 40.09 |
| Na2O | 0.21 | 1.37 |
| K2O | 0.14 | 0.39 |
| TiO2 | 0.08 | 0.13 |
| P2O5 | 8.41 | 9.95 |
| LOI | 23.22 | 15.91 |
| Total | 89.07 | 79.06 |
| Element | FL | EH | Element | FL | EH |
|---|---|---|---|---|---|
| Be | 2 | 2 | Mo | 15 | 14 |
| V | 262 | 582 | Ag | 6.3 | b.d.l. |
| Cr | 330 | 730 | In | b.d.l. | b.d.l. |
| Co | b.d.l. | b.d.l. | Sn | b.d.l. | 1 |
| Ni | 130 | 80 | Sb | 2.5 | 10.8 |
| Cu | 170 | 70 | Cs | 0.6 | 1.9 |
| Zn | 810 | 220 | Ba | 934 | 153 |
| Ga | 3 | 6 | Bi | b.d.l. | b.d.l. |
| Ge | b.d.l. | b.d.l. | Hf | 2.4 | 1.9 |
| As | b.d.l. | 22 | Ta | 0.6 | 1.3 |
| Rb | 7 | 13 | W | 4 | 4 |
| Sr | 880 | 313 | Tl | 0.2 | b.d.l. |
| Zr | 133 | 120 | Pb | 5 | 10 |
| Nb | 5 | 3 |
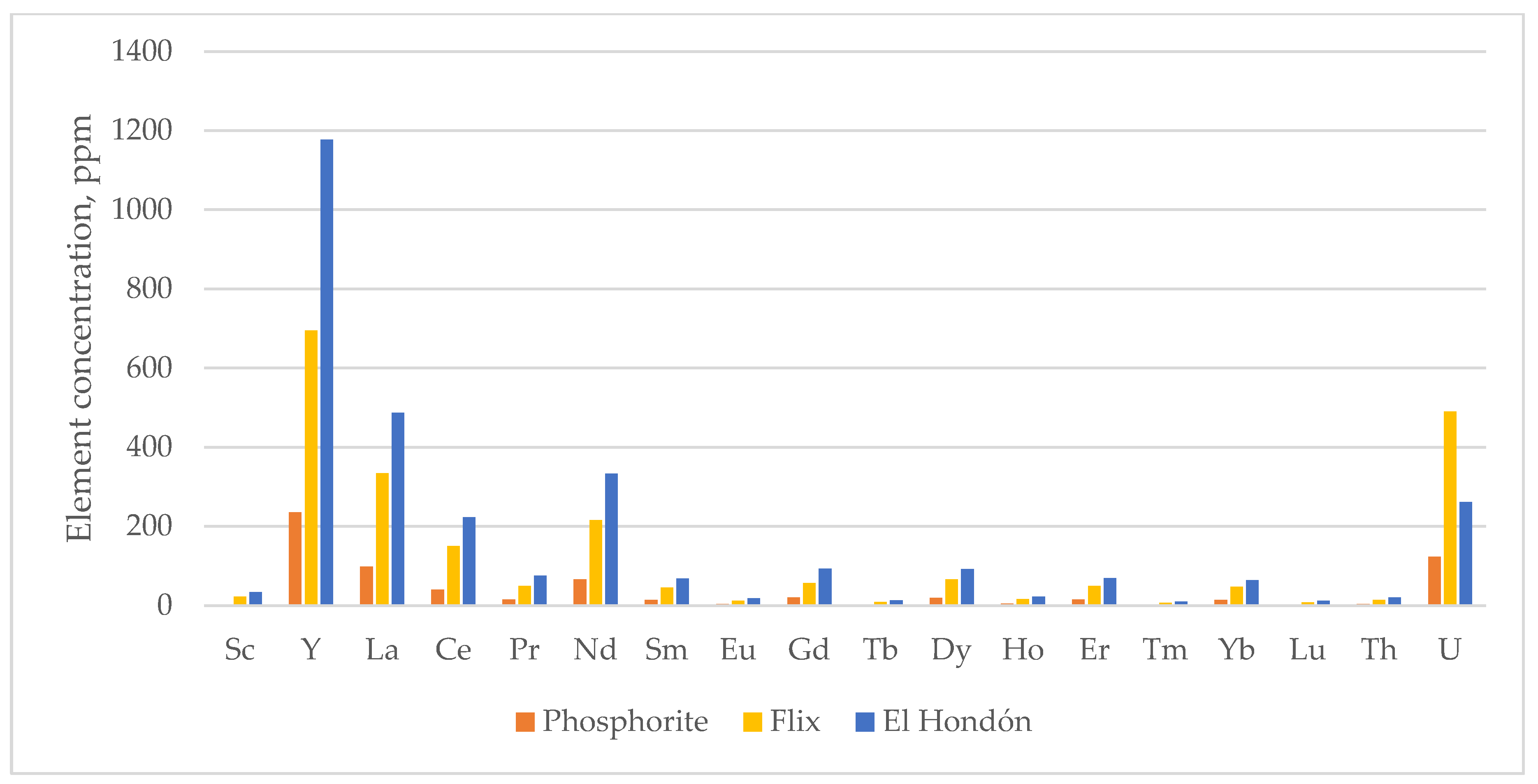
| Element | Sc | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Th | U |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1_1 | 34 | 1176 | 484 | 188 | 73 | 318 | 65 | 17 | 85 | 14 | 95 | 22 | 68 | 9 | 63 | 11 | 19 | 299 |
| A1_2 | nd | 1220 | 492 | 174 | 70 | 315 | 65 | 17 | 95 | 13 | 91 | 22 | 74 | 11 | 67 | 11 | 19 | 165 |
| A1_3 | nd | 762 | 285 | 107 | 43 | 194 | 39 | 10 | 56 | 8 | 55 | 13 | 43 | 6 | 40 | 7 | 8 | 141 |
| A3_1 | 31 | 1140 | 446 | 162 | 66 | 281 | 58 | 15 | 75 | 12 | 83 | 20 | 64 | 9 | 62 | 11 | 16 | 293 |
| A3_2 | nd | 1435 | 577 | 219 | 85 | 382 | 78 | 20 | 109 | 15 | 107 | 26 | 83 | 12 | 77 | 13 | 21 | 287 |
| A12 | 35 | 1209 | 495 | 198 | 75 | 324 | 67 | 18 | 87 | 13 | 95 | 22 | 70 | 10 | 66 | 12 | 21 | 253 |
| B2_1 | 33 | 1169 | 471 | 195 | 73 | 315 | 66 | 17 | 84 | 13 | 92 | 22 | 70 | 10 | 66 | 12 | 23 | 249 |
| B2_2 | nd | 1105 | 641 | 704 | 124 | 539 | 106 | 25 | 119 | 16 | 101 | 23 | 70 | 10 | 58 | 9 | 57 | 164 |
| B3_1 | 35 | 1340 | 528 | 226 | 86 | 369 | 78 | 20 | 96 | 15 | 106 | 24 | 74 | 10 | 68 | 12 | 23 | 212 |
| B3_2 | nd | >1000 | 475 | 194 | 72 | 350 | 65 | 17 | 100 | 13 | 94 | 24 | 70 | 11 | 67 | nd | 18 | 210 |
| B3_3 | nd | >1000 | 464 | 203 | 82 | 320 | 63 | 20 | 107 | 13 | 100 | 23 | 71 | 10 | 68 | nd | 19 | 215 |
| B3_4 | nd | 1250 | 507 | 181 | 73 | 332 | 67 | 18 | 98 | 14 | 94 | 23 | 73 | 11 | 67 | 11 | 17 | 245 |
| B3_5 | nd | 1145 | 465 | 176 | 68 | 306 | 61 | 16 | 89 | 12 | 85 | 21 | 68 | 10 | 61 | 10 | 17 | 196 |
| B4 | nd | 1320 | 526 | 220 | 76 | 346 | 70 | 18 | 103 | 14 | 96 | 24 | 75 | 11 | 68 | 12 | 21 | 294 |
| B5 | nd | 1030 | 450 | 203 | 70 | 309 | 65 | 17 | 88 | 12 | 82 | 19 | 61 | 9 | 55 | 9 | 22 | 247 |
| AVG EH | 34 | 1177 | 487 | 223 | 76 | 333 | 68 | 18 | 93 | 13 | 92 | 22 | 69 | 10 | 64 | 11 | 21 | 231 |
| FL | 22 | 695 | 334 | 150 | 49 | 216 | 45 | 12 | 57 | 9 | 65 | 16 | 49 | 7 | 47 | 8 | 14 | 490 |
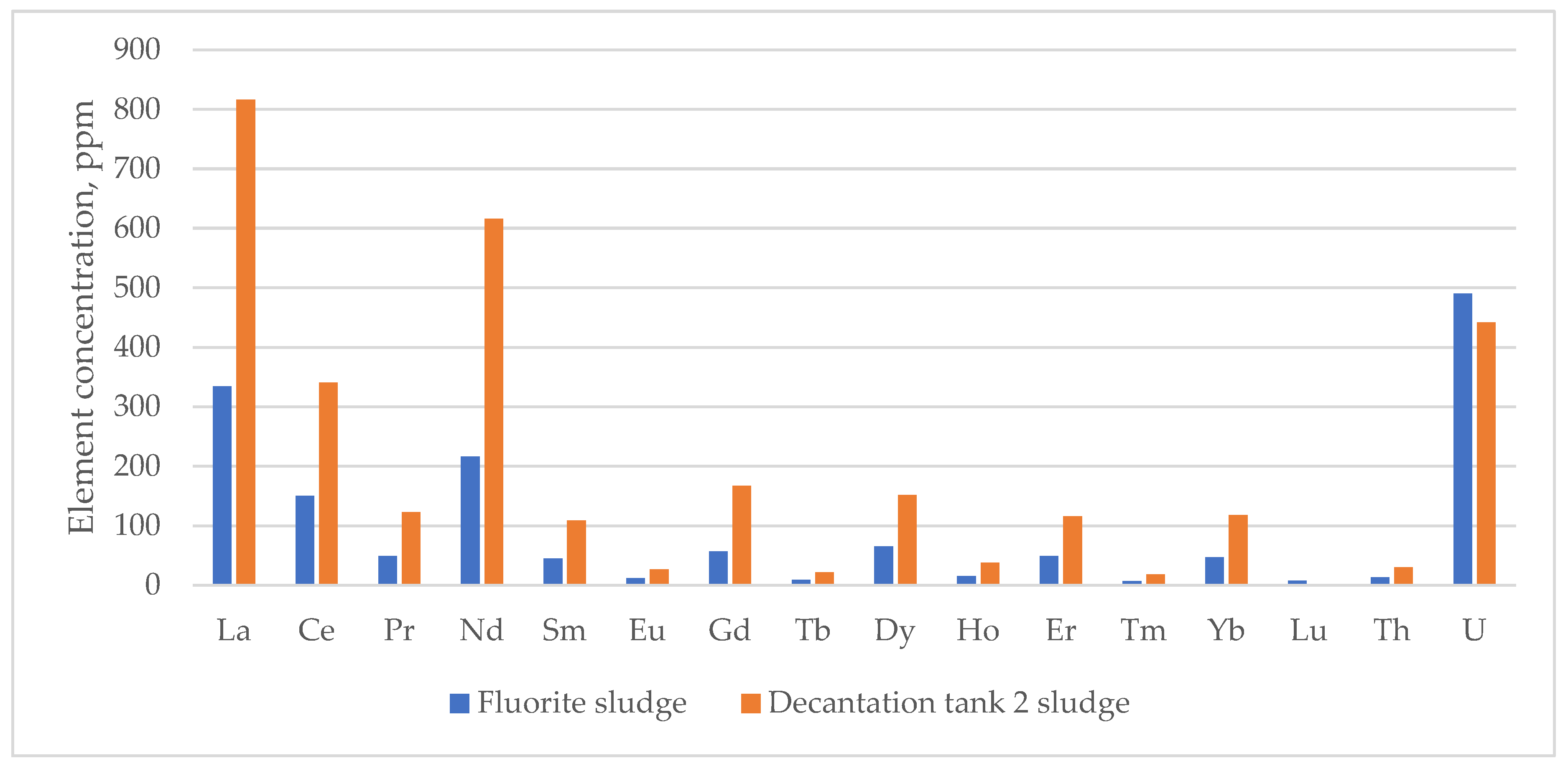
| T1_1 | nd | 871 | 335 | 142 | 60 | 224 | 42 | 12 | 76 | 10 | 66 | 17 | 49 | 7 | 50 | nd | 12 | 309 |
| T1_2 | nd | 817 | 318 | 132 | 48 | 226 | 46 | 13 | 67 | 8 | 58 | 15 | 47 | 7 | 47 | nd | 11 | 312 |
| T2_1 | nd | >1000 | 765 | 333 | 133 | 516 | 109 | 27 | 166 | 21 | 161 | 38 | 117 | 16 | 114 | nd | 28 | 400 |
| T2_2 | nd | >1000 | 816 | 341 | 123 | 616 | 109 | 27 | 167 | 22 | 152 | 38 | 116 | 19 | 118 | nd | 30 | 442 |
| T3_1 | nd | >1000 | 690 | 301 | 120 | 452 | 100 | 27 | 143 | 20 | 146 | 34 | 103 | 15 | 99 | nd | 25 | 428 |
| T3_2 | nd | >1000 | 697 | 313 | 120 | 472 | 98 | 26 | 158 | 20 | 147 | 33 | 109 | 14 | 108 | nd | 25 | 429 |
| PR | nd | 235 | 98 | 40 | 15 | 66 | 14 | 4 | 20 | 3 | 19 | 5 | 15 | 2 | 13 | 2 | 3 | 124 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płachciak, M.; Grandia, F.; Roddatis, V.; Syczewski, M.D. Characterization of Waste from the Dicalcium Phosphate Industry as a Potential Secondary Source of Rare Earth Elements. Mater. Proc. 2023, 15, 34. https://doi.org/10.3390/materproc2023015034
Płachciak M, Grandia F, Roddatis V, Syczewski MD. Characterization of Waste from the Dicalcium Phosphate Industry as a Potential Secondary Source of Rare Earth Elements. Materials Proceedings. 2023; 15(1):34. https://doi.org/10.3390/materproc2023015034
Chicago/Turabian StylePłachciak, Marcin, Fidel Grandia, Vladimir Roddatis, and Marcin Daniel Syczewski. 2023. "Characterization of Waste from the Dicalcium Phosphate Industry as a Potential Secondary Source of Rare Earth Elements" Materials Proceedings 15, no. 1: 34. https://doi.org/10.3390/materproc2023015034
APA StylePłachciak, M., Grandia, F., Roddatis, V., & Syczewski, M. D. (2023). Characterization of Waste from the Dicalcium Phosphate Industry as a Potential Secondary Source of Rare Earth Elements. Materials Proceedings, 15(1), 34. https://doi.org/10.3390/materproc2023015034






