1. Introduction
Used medical technology products are wastes that are considered to be contaminated, and for this reason, their management and disposal is governed by many regulations and laws, and their reuse (of those that could be reused) is generally avoided or prohibited. The application of minimally invasive medical techniques requires the use of specialized equipment, such as catheters, guide wires and stents, which constitute a large part of the total waste of a respective facility (i.e., a hospital) that finally ends up in landfills.
Complying with the circular economy framework, efforts and research have been conducted worldwide to develop methods to reuse these products, thus reducing the need of raw materials and, possibly, reducing costs [
1]. Furthermore, a methodology for their management and collection should be developed, with the aim of exploiting their manufacturing materials and, especially, the precious metals they contain.
Noble metals (Pt, Ir, Au, Ta, etc.) have found several applications in specific medical technology products since they present inertness and visibility when examined via fluoroscopy by an external observer/operator [
2]. These products are usually applied as single-use materials and, as a result, the corresponding waste streams may be quite rich in valuable metals. On the other hand, limited research has been performed thus far regarding the recovery of these precious metals from such products [
3].
The aim of this study was to provide a detailed structural/chemical characterization of the respective raw materials used in medical technology products and the development of a hydrometallurgical methodology to dissolve them so that the contained noble metals could be subsequently recovered (possibly selectively) from the respective solutions. The common medical products examined in this case were an electrophysiology catheter (Ir/Pt), a diagnostic guide wire (Au) and a stent (Ta).
2. Materials and Methods
This study was carried out using new (unused) commercially available samples, namely an electrophysiology catheter (Ir/Pt) manufactured by Boston Scientific, model Viking; a diagnostic guide wire (Au) manufactured by ev3 company, model Nitrex; and a stent (Ta) manufactured by ev3 company, model Protege. The parts that contain the noble metals of interest were separated by cutting them. Afterwards, they were placed in a porcelain capsule with a lid and an exhaust gas outlet hole to pyrolyze them. The thermal treatment was conducted in the range of 500–550 °C using a WITEG Muffle Furnace, model FHX-05.
A Field-Emission Scanning Electron Microscope (FE-SEM Quanta 200, Berkhamsted, UK), equipped with an EDAX energy-dispersive X-ray spectroscopy analyzer, was used to determine the distribution of elements in the structure of the examined samples.
Dissolution experiments were carried out in a beaker under reflux using a hot plate. Different mixtures of inorganic acids were tested, such as HNO3, HCl, HF and H2SO4. The efficiency of the hydrometallurgical process was evaluated according to the complete and selective solubilization of the metals Au, Pt, Ir and Ta (100% of them in liquid phase), while other potential impurities, e.g., Fe, Cr, Ni and Ti, were not taken into account.
3. Results
3.1. Pyrolysis
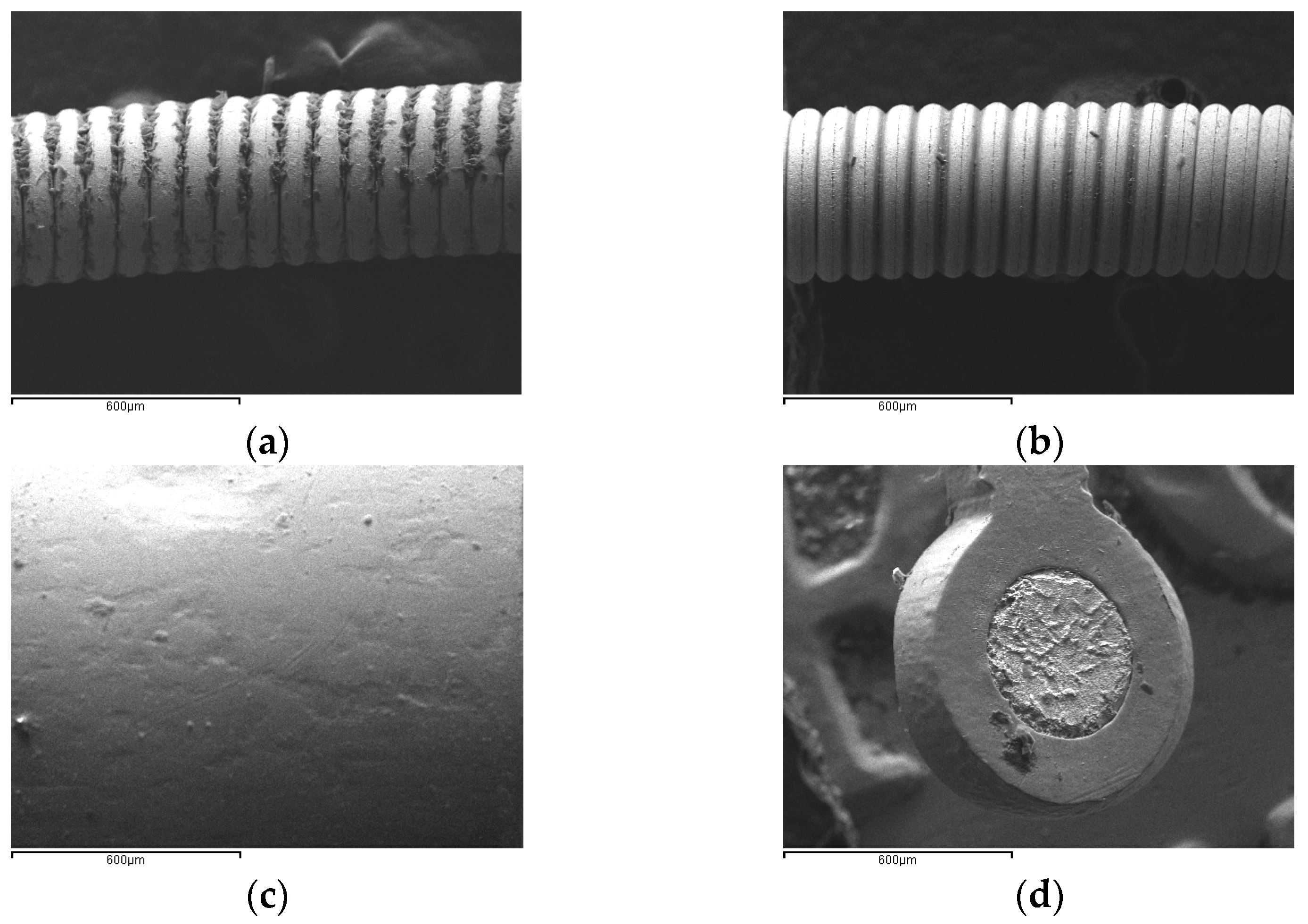
The optimization of pyrolysis temperature was conducted by using the guide wire sample as the reference material. This selection was based on the fact that it is the only case of the examined samples where plastic parts overlap the point containing a metal of interest, i.e., Au. According to
Figure 1a, at 500 °C, a high loss of the plastic parts was achieved and deposits of tar were obtained. But at 550 °C, these deposits were eliminated (
Figure 1b) [
4]. Moreover, the SEM images of the gold-plated area show the presence of a lamina with spiral geometry and relatively good uniformity.
The surface morphology of the Pt/Ir bands appears to be quite smooth with few surface scratches due to their metallurgical production process (
Figure 1c). These bands are placed on the plastic wire containing a stainless-steel grid. The next sample is a Ta stent consisting of a Ni/Ti alloy grid, where Ta spots are homogeneously dispersed (
Figure 1d).
3.2. Chemical Analysis
Energy-dispersive X-ray spectroscopy (EDAX) is a microanalysis technique used for identifying elements present in the examined samples. This technique also quantifies the elemental composition of the samples. According to
Table 1, all the examined samples are composed of high-purity noble metals. In the initial/commercial products, a notable percentage of oxygen was obtained, indicating that these noble metals were partially oxidized. Pyrolysis led to the reduction, up to zero, of oxygen for the catheter and the guide wire. On the other hand, an increase was observed in the stent sample, indicating further Ta oxidation [
5].
3.3. Dissolution
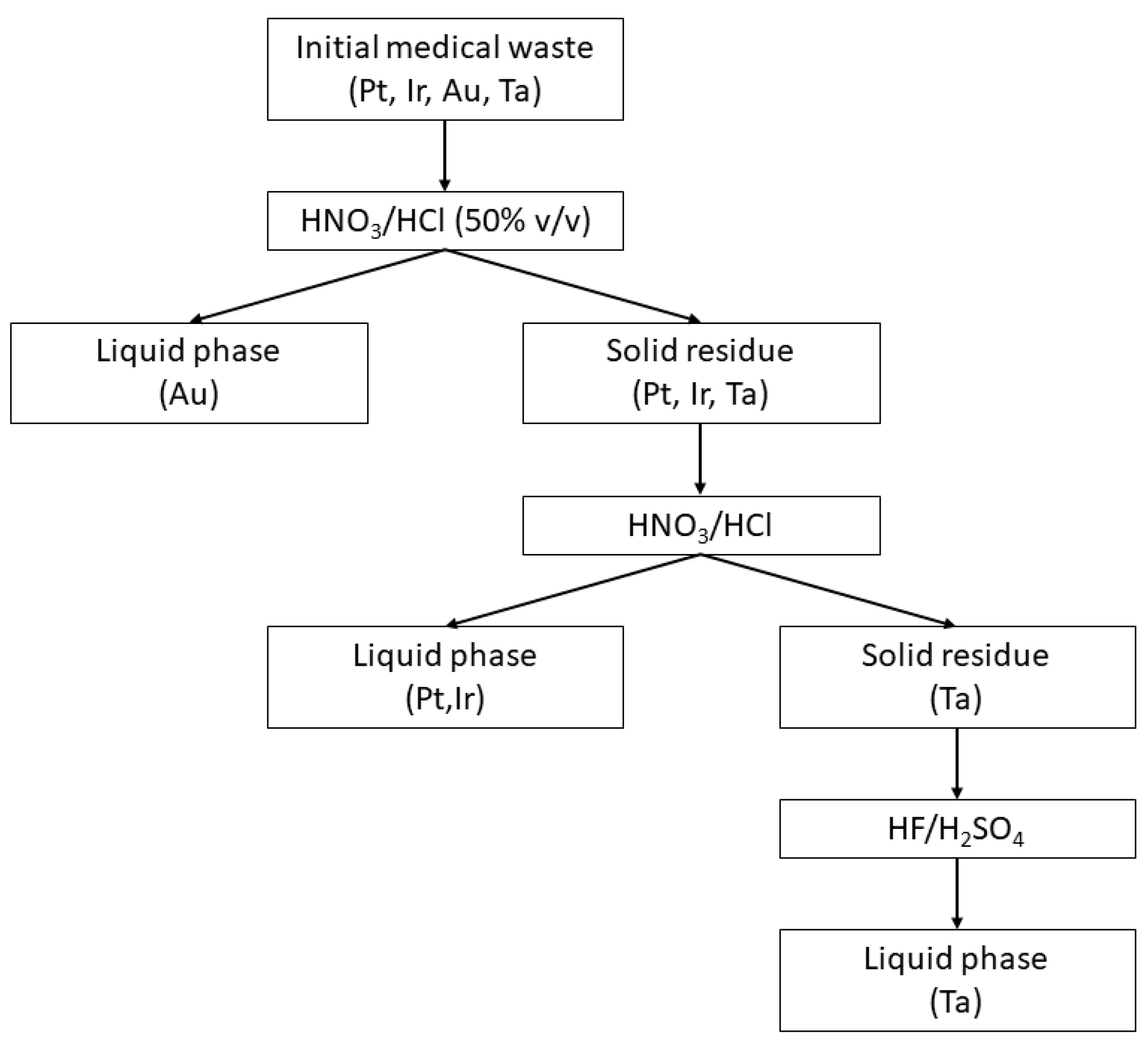
The examined medical products were considered to be single and mixed waste streams, simulating realistic conditions.
Figure 2 presents the proposed selective recovery method for recovering noble metals. Initially, a 50%
v/
v solution of aqua regia (HNO
3/HCl) was added in the plastic-free/pyrolyzed wastes. Under mild heating conditions (50 °C), Au dissolution was achieved almost instantly (100% of Au in liquid phase) [
6].
The Au-rich liquid phase was separated, and concentrated aqua regia was added to the solid residue. Pt and Ir dissolution required boiling conditions (100–120 °C) and multiple additions of aqua regia (100% of Pt/Ir in liquid phase) [
7]. Despite the use of the same acidic reagent, the solubility differences of Pt/Ir and Au led to selective extraction from the waste stream. On the other hand, Ta dissolution (100% of Ta in liquid phase) required the addition, mainly, of HF (6N) and H
2SO
4 (8N) since it was insoluble in the aqua regia solutions and under the temperature range that were previously applied [
8].
4. Conclusions
According to the proposed methodology, the first step (after disinfection) requires cutting (separating) the specific parts of medical products that contain mainly noble metals to increase their content. Afterwards, pyrolysis is applied so that plastic coverings/organic residues are removed completely. The selective dissolution of the examined noble metals is followed by using mixtures of inorganic acids under different experimental conditions. Through this work, it was proven that separate flows of the metals of interest can be obtained, and the sufficient recovery of these metals can be achieved for potential reuse in similar (or other) applications after separating them from any impurities. Thus, as a future work, the selective separation of the metals of interest from other potential impurities in each liquid phase is strongly recommended.
Author Contributions
Conceptualization, Ε.Κ., D.M. and A.Z.; methodology, E.K., D.M. and C.P.; software, K.S.; validation, Ε.Κ., G.V. and A.Z.; formal analysis, E.P.; investigation, E.K. and A.L.; resources, A.Z.; data curation, E.K., K.S., D.M. and A.L.; writing—original draft preparation, E.K. and C.P.; writing—review and editing, Ε.Κ., E.P. and A.Z.; visualization, E.K. and K.S.; supervision, G.V. and A.Z.; project administration, E.P.; funding acquisition, A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The project «Collection, processing and metallurgical recovery of critical raw materials (Au, Pt, Ir, Ta) from discarded medical material» (Project code: ΚΜΡ6-0084436) was implemented under the framework of the Action «Investment Plans of Innovation» of the Operational Program «Central Macedonia 2014 2020», which is co-funded by the European Regional Development Fund in partnership agreement with Greece.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kapoor, A.; Vora, A.; Nataraj, G.; Mishra, S.; Kerkar, P.; Manjunath, C.N. Guidance on reuse of cardio-vascular catheters and devices in India: A consensus document. Indian Heart J. 2017, 69, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cai, W.; Li, H.T.; Zheng, Y.F. Surface modification of NiTi alloy with C to improve its biocompatibility and radiopacity. J. Mater. Sci. 2006, 41, 4961–4964. [Google Scholar] [CrossRef]
- Guimarães, R.; Carvalho, J.; Leal, V.; Guerner Dias, A.J. Characterization, treatment proposal and metal recovery in waste of active implantable medical devices. Comun. Geol. 2014, 101, 1011–1014. [Google Scholar]
- Su, G.; Ong, H.C.; Ibrahim, S.; Fattah, I.M.R.; Mofijur, M.; Chong, C.T. Valorisation of medical waste through pyrolysis for a cleaner environment: Progress and challenges. Environ. Pollut. 2021, 279, 116934. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, R.; Park, I.; Masel, R.I.; Shannon, M.A. Thermal oxidation of tantalum films at various oxidation states from 300 to 700 °C. J. Appl. Phys. 2005, 98, 114908. [Google Scholar] [CrossRef]
- Rao, M.D.; Singh, K.K.; Morrison, C.A.; Love, J.B. Challenges and opportunities in the recovery of gold from electronic waste. RSC Adv. 2020, 10, 4300–4309. [Google Scholar] [CrossRef] [PubMed]
- Schreier, G.; Edtmaier, C. Separation of Ir, Pd and Rh from secondary Pt scrap by precipitation and calcination. Hydrometallurgy 2003, 68, 69–75. [Google Scholar] [CrossRef]
- Shikika, A.; Sethurajan, M.; Muvundja, F.; Mugumaoderha, M.C.; Gaydardzhiev, S. A review on extractive metallurgy of tantalum and niobium. Hydrometallurgy 2020, 198, 105496. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).