Characterization and Comparison of WO3 with Hybrid WO3-MoO3 and TiO2 with Hybrid TiO2-ZnO Nanostructures as Photoanodes †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Nanostructures
2.2. Morphological and Crystalline Characterization
2.3. Photoelectrochemical Properties
3. Results and Discussion
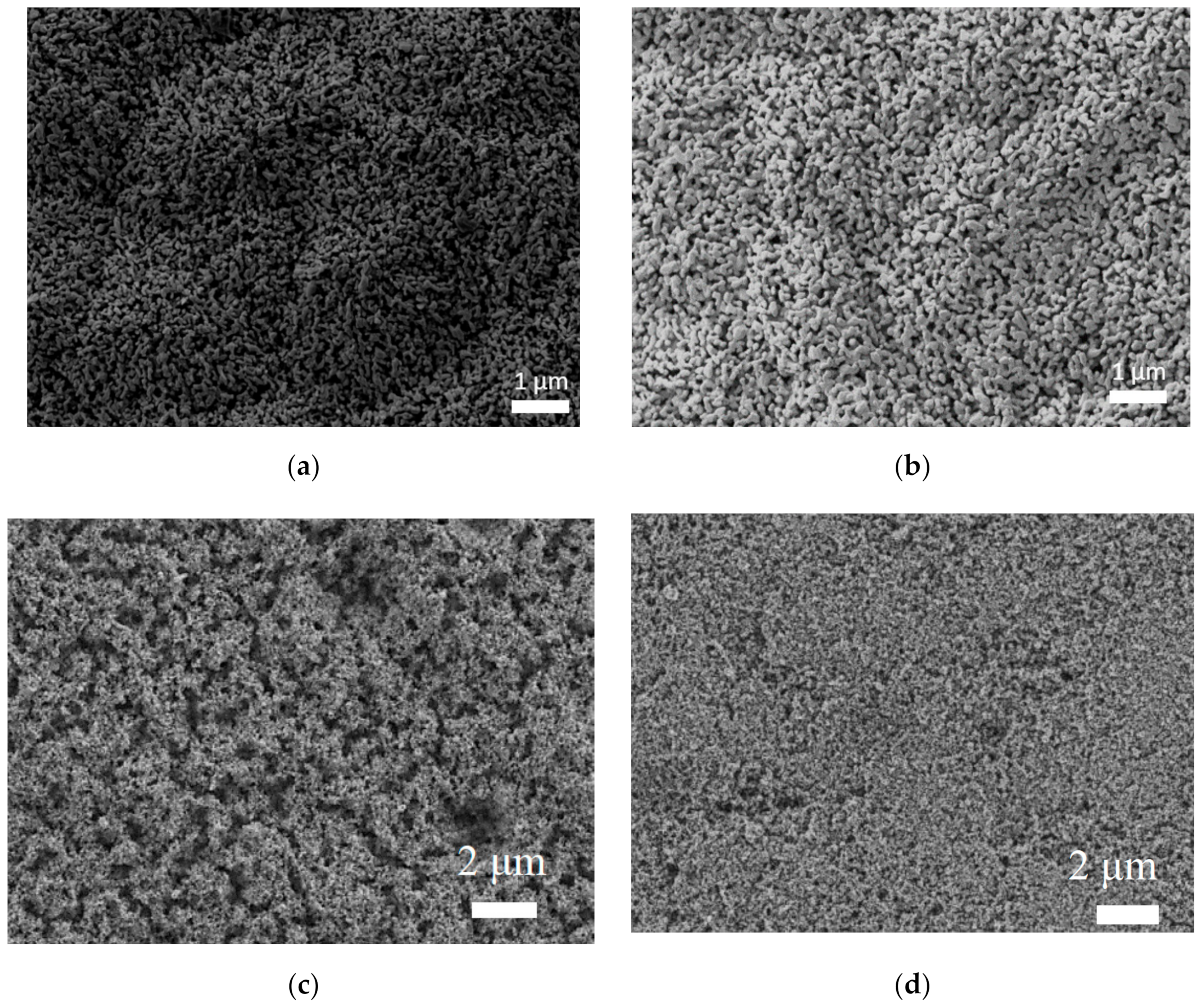
3.1. FE-SEM
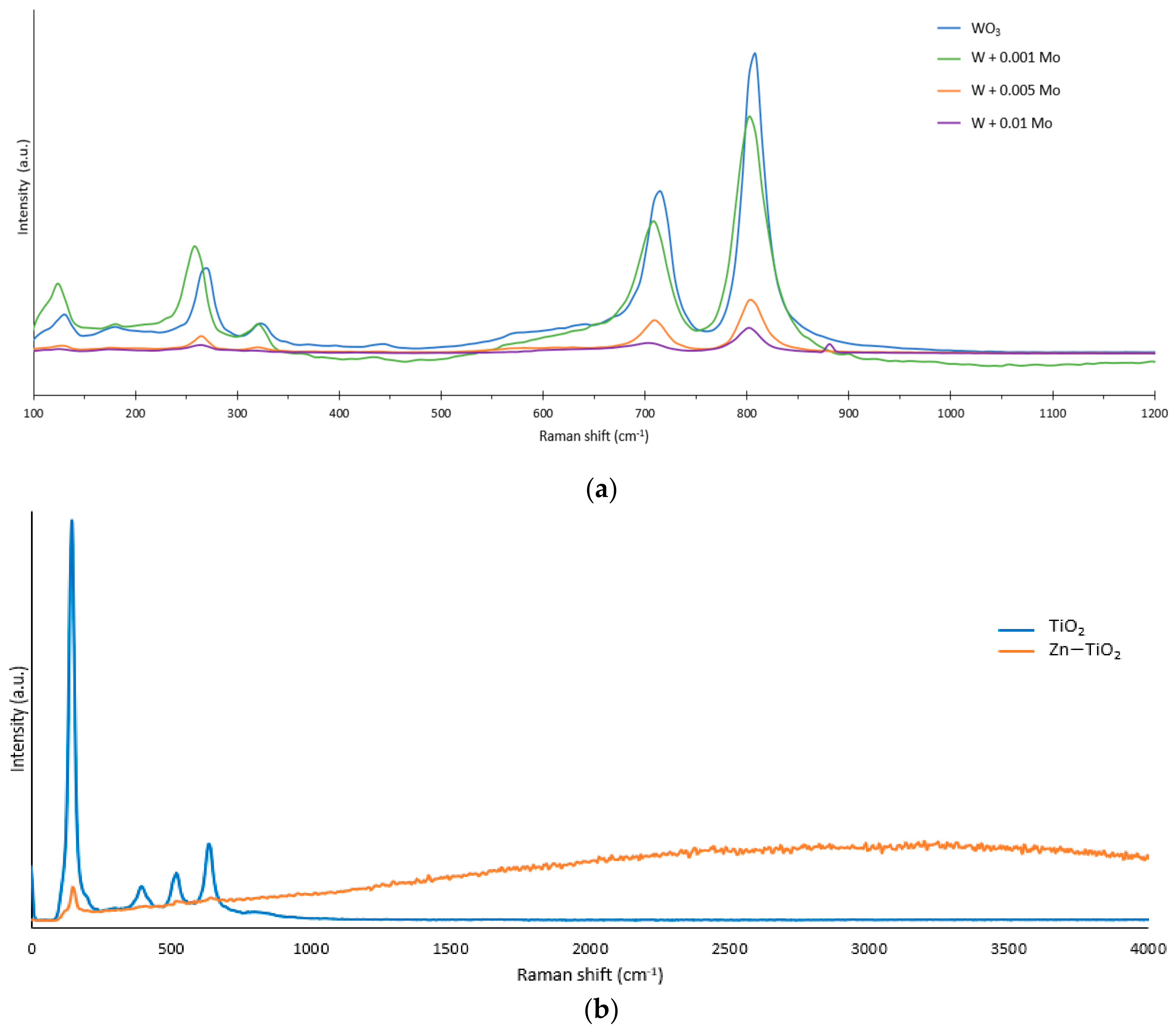
3.2. Raman
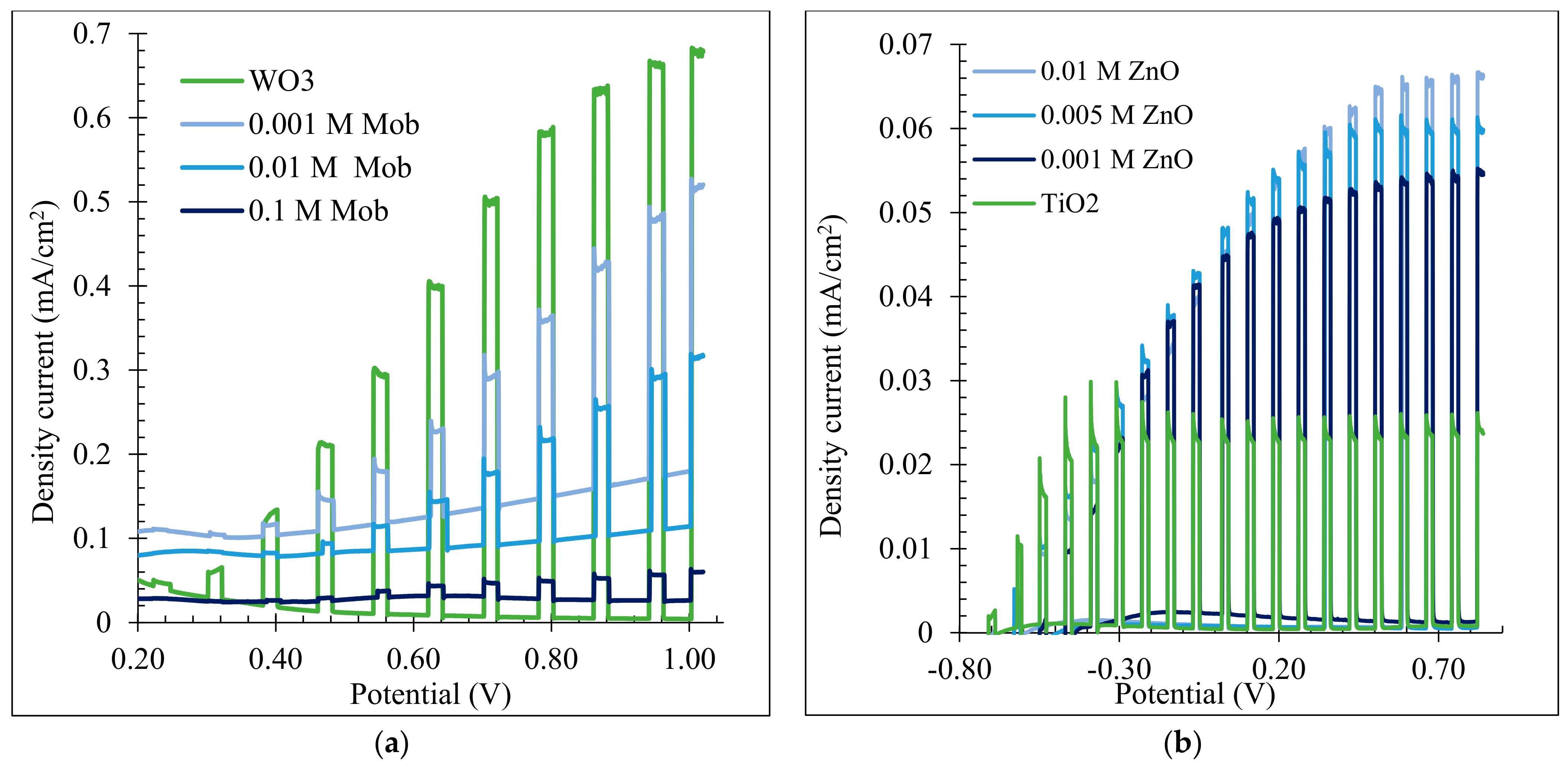
3.3. Water Splitting Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, J.-P.; Urbain, F.; Smirnov, V.; Rau, U.; Ziegler, J.; Kaiser, B.; Jaegermann, W.; Finger, F. Modeling and Practical Realization of Thin Film Silicon-Based Integrated Solar Water Splitting Devices. Phys. Status Solidi 2016, 213, 1738–1746. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Wang, Q. Nanostructure-Based WO3 Photoanodes for Photoelectrochemical Water Splitting. Phys. Chem. Chem. Phys. 2012, 14, 7894–7911. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Teng, H.; Zhang, L.; Zhou, J.; Liu, M. Synthesis of Mo-Doped WO3 Nanosheets with Enhanced Visible-Light-Driven Photocatalytic Properties. RSC Adv. 2015, 5, 95394–95400. [Google Scholar] [CrossRef]
- Harris, J.; Silk, R.; Smith, M.; Dong, Y.; Chen, W.-T.; Waterhouse, G.I.N. Hierarchical TiO2 Nanoflower Photocatalysts with Remarkable Activity for Aqueous Methylene Blue Photo-Oxidation. ACS Omega 2020, 5, 18919–18934. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Gazquez, P.J.; Muñoz-Portero, M.J.; Blasco-Tamarit, E.; Sánchez-Tovar, R.; Fernández-Domene, R.M.; García-Antón, J. Original Approach to Synthesize TiO2/ZnO Hybrid Nanosponges Used as Photoanodes for Photoelectrochemical Applications. Materials 2021, 14, 6441. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A Review on Highly Ordered, Vertically Oriented TiO2 Nanotube Arrays: Fabrication, Material Properties, and Solar Energy Applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Cifre-Herrando, M.; Roselló-Márquez, G.; García-García, D.M.; García-Antón, J. Degradation of Methylparaben Using Optimal WO3 Nanostructures: Influence of the Annealing Conditions and Complexing Agent. Nanomaterials 2022, 12, 4286. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Domene, R.M.; Sánchez-Tovar, R.; Sánchez-González, S.; Garcia-Anton, J. Photoelectrochemical Characterization of Anatase-Rutile Mixed TiO2 Nanosponges. Int. J. Hydrogen Energy 2016, 41, 18380–18388. [Google Scholar] [CrossRef]
- Borràs-Ferrís, J.; Sánchez-Tovar, R.; Blasco-Tamarit, E.; Fernández-Domene, R.M.; Garcia-Anton, J. Effect of Reynolds Number and Lithium Cation Insertion on Titanium Anodization. Electrochim. Acta 2016, 196, 24–32. [Google Scholar] [CrossRef]
- Jittiarporn, P.; Sikong, L.; Kooptarnond, K.; Taweepreda, W.; Stoenescu, S.; Badilescu, S.; Truong, V. Van Electrochromic Properties of MoO3-WO3 Thin Films Prepared by a Sol-Gel Method, in the Presence of a Triblock Copolymer Template. Surf. Coat. Technol. 2017, 327, 66–74. [Google Scholar] [CrossRef]
| Concentration of Mob (M) | % (Weight) | % (Atomic) | Concentration of Zn(NO3)2 (M) | % Weight | % Atomic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | W | M | O | W | M | O | Ti | Zn | O | Ti | Zn | ||
| 0 | 18.02 | 81.98 | 0.00 | 71.66 | 28.33 | 0.00 | 0 | 34.51 | 65.49 | 0.00 | 61.21 | 38.79 | 0.00 |
| 0.001 | 28.77 | 70.32 | 0.92 | 82.10 | 17.46 | 0.44 | 0.001 M | 38.11 | 57.56 | 4.33 | 65.26 | 32.92 | 1.82 |
| 0.005 | 19.40 | 78.88 | 1.71 | 73.07 | 25.85 | 1.08 | 0.005 M | 36.07 | 58.08 | 5.85 | 63.39 | 34.10 | 2.51 |
| 0.01 | 18.01 | 80.02 | 1.97 | 71.18 | 27.53 | 1.30 | 0.01 M | 35.04 | 57.81 | 7.15 | 62.45 | 34.43 | 3.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cifre-Herrando, M.; Roselló-Márquez, G.; Navarro-Gázquez, P.J.; Muñoz-Portero, M.J.; Blasco-Tamarit, E.; García-Antón, J. Characterization and Comparison of WO3 with Hybrid WO3-MoO3 and TiO2 with Hybrid TiO2-ZnO Nanostructures as Photoanodes. Mater. Proc. 2023, 14, 69. https://doi.org/10.3390/IOCN2023-14487
Cifre-Herrando M, Roselló-Márquez G, Navarro-Gázquez PJ, Muñoz-Portero MJ, Blasco-Tamarit E, García-Antón J. Characterization and Comparison of WO3 with Hybrid WO3-MoO3 and TiO2 with Hybrid TiO2-ZnO Nanostructures as Photoanodes. Materials Proceedings. 2023; 14(1):69. https://doi.org/10.3390/IOCN2023-14487
Chicago/Turabian StyleCifre-Herrando, M., G. Roselló-Márquez, Pedro José Navarro-Gázquez, María José Muñoz-Portero, E. Blasco-Tamarit, and J. García-Antón. 2023. "Characterization and Comparison of WO3 with Hybrid WO3-MoO3 and TiO2 with Hybrid TiO2-ZnO Nanostructures as Photoanodes" Materials Proceedings 14, no. 1: 69. https://doi.org/10.3390/IOCN2023-14487
APA StyleCifre-Herrando, M., Roselló-Márquez, G., Navarro-Gázquez, P. J., Muñoz-Portero, M. J., Blasco-Tamarit, E., & García-Antón, J. (2023). Characterization and Comparison of WO3 with Hybrid WO3-MoO3 and TiO2 with Hybrid TiO2-ZnO Nanostructures as Photoanodes. Materials Proceedings, 14(1), 69. https://doi.org/10.3390/IOCN2023-14487






