Abstract
Gold nanoparticles (AuNPs) are one of the most remarkable nanomaterials. Due to their small size, these NPs can cross the blood–brain barrier making them good candidates for the treatment of diseases related to the central nervous system. The main objective of the present work was to evaluate the influence of surface charge on the biological behaviour of AuNPs by assessing the cytotoxic—viability and morphological alterations—and genotoxic—double strand breaks—effects induced in neuronal cells exposed to AuNPs with different charges: cationic, anionic, and neutral. Different toxicological behaviours were obtained depending on the surface charge of the NPs.
1. Introduction
Gold nanoparticles (AuNPs) are one of the most remarkable nanomaterials. They have aroused great interest in recent years because of their particular properties and their high potential for biomedical applications [1,2]. Due to their small size, these NPs can cross the blood–brain barrier, which makes them good candidates for the treatment of diseases related to the central nervous system [3,4]. Despite these potential benefits, the information about the short- and long-term effects of AuNPs in organisms and the environment is very scarce, although several adverse effects have been reported (reviewed in [5,6]). Once AuNPs enter the body, their interactions with biological systems have been found to be related to their physicochemical properties, which determine their internalization within cells [5]. The main physicochemical properties that affect AuNP toxicity include size, surface chemistry, and shape [7]. On this basis, the main objective of the present work was to evaluate the influence of surface charge on the biological behaviour of AuNPs. Thus, the cytotoxic and genotoxic effects induced by AuNPs with different charges, i.e., cationic, anionic, and neutral, were assessed in neuronal SH-SY5Y cells.
2. Materials and Methods
The three types of AuNPs used in the present study were newly synthesized following the method reported by Brust et al. [8]. The average hydrodynamic size and zeta potential of NPs in neuron culture medium were determined by dynamic light scattering (DLS) and electrophoretic light scattering (ELS), respectively, using a Zetasizer Nano-ZS (model ZEN 3600, Malvern Instruments Lt, Malvern, UK).
Morphological analysis was performed by employing an inverted light microscope (Nikon Instruments Inc., Melville, NY, USA). Phase-contrast photographs of control and AuNP-treated cells were obtained. The NP effects on viability were evaluated by MTT assay [9] using a SPECTROstar Nano (BMG Labtech, Ortenberg, Germany) microplate reader, and analysis of H2AX phosphorylation was carried out by flow cytometry [10] in a FACScalibur cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). For all these experiments, SH-SY5Y cells were incubated with the three different AuNPs at a range of concentrations or the control solutions for 3 or 24 h.
Differences among groups were statistically analysed by the Kruskal–Wallis test, and Mann–Whitney U-test for two-by-two comparisons, by employing the SPSS for Windows statistical package (version 20.0, IBM, Armonk, NY, USA). The associations between two variables were analysed by Pearson’s correlation. The experimental data are expressed as mean ± standard error and a p-value of <0.05 was considered significant. All experiments were run at least in triplicate.
3. Results and Discussion
3.1. Nanoparticle Characterization and Cellular Uptake
The AuNPs employed in the present study are 2–4 nm spherical NPs with a positive (cationic), negative (anionic), or neutral surface charge. The results obtained from the analysis of the hydrodynamic size and zeta potential of these NPs are collected in Table 1. The dispersions of the AuNPs were quite stable and similar between al NPs, with almost no variations in the hydrodynamic sizes. The zeta potential values confirmed the charge of the coating of the NPs and supported their stability in a suspension.

Table 1.
Physical–chemical characterization of AuNP.
3.2. Morphological Alterations after AuNP Exposure
No morphological changes in the neuronal cells were found after treatment with anionic or cationic AuNPs for the selected exposure times. In the case of neutral AuNPs, morphological alterations were only detected after 24 h of exposure at the highest concentrations and included rounding of the cells, loss of neurites, and slight detaching from the surface. Example photomicrographs are shown in Figure 1.
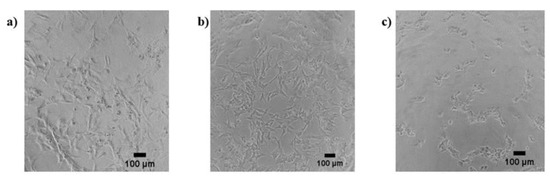
Figure 1.
SH-SY5Y neuronal cells without treatment: (a) and treated with 0.5 µg/mL (b) and 50 µg/mL (c) of neutral AuNPs.
3.3. Viability of Neuronal Cells Exposed to AuNPs
The effects of AuNP exposure on the viability of neuronal SH-5YSY cells were evaluated using the MTT assay. Following Costa et al. [11], a modified MTT protocol was employed to avoid any potential interference of the NPs. The results from these experiments are shown in Figure 2, Figure 3 and Figure 4. Although slight but significant decreases in viability were observed for anionic AuNP treatments (Figure 2), they cannot be considered cytotoxic effects according to ISO 10993-5 [12], since the reductions in cell viability were not higher than 30%.
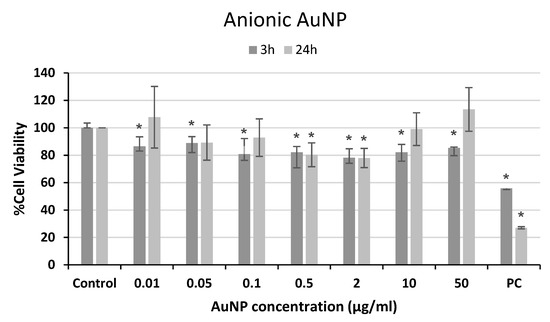
Figure 2.
Cell viability of human neuroblastoma SH-SY5Y cells after exposure to anionic AuNPs for 3 and 24 h. PC: positive control (1% Triton 100-X). * p < 0.05, significant difference compared to the corresponding negative control.
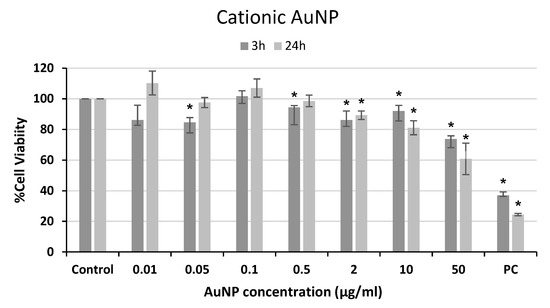
Figure 3.
Cell viability of human neuroblastoma SH-SY5Y cells after exposure to cationic AuNPs for 3 and 24 h. PC: positive control (1% Triton 100-X). * p < 0.05, significant difference compared to the corresponding negative control.
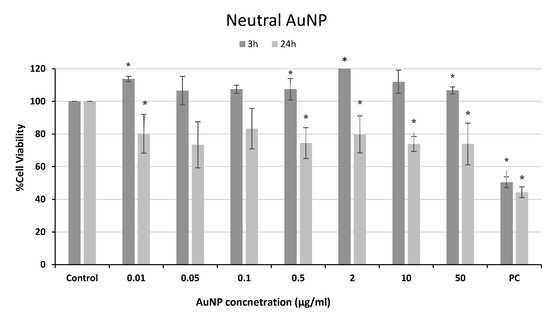
Figure 4.
Cell viability of human neuroblastoma SH-SY5Y cells after exposure to neutral AuNPs for 3 and 24 h. PC: positive control (1% Triton 100-X). * p < 0.05, significant difference compared to the corresponding negative control.
A decrease in cell viability was observed after cationic AuNP treatment at the highest doses with both exposure times (Figure 3), reaching values of 70% and 60% viability at the highest concentration employed after 3 or 24 h, respectively. However, a statistically significant dose–response relationship was only found for the 24 h treatment (r = −0.795; p < 0.01).
For neutral AuNP exposure, significant decreases in cellular viability compared to the negative control were found only after 24 h of treatment, with values around 70–80% at all concentrations tested (Figure 4).
3.4. Genotoxic Effects of AuNP
The results obtained from the analysis of H2AX phosphorylation of neuronal SH-SY5Y cells exposed to anionic, cationic, or neutral AuNP are shown in Figure 5, Figure 6 and Figure 7. Slight increases were observed in the percentage of cells with γH2AX at all the concentrations tested for anionic AuNPs, although the values registered always maintained below 10% (Figure 5).
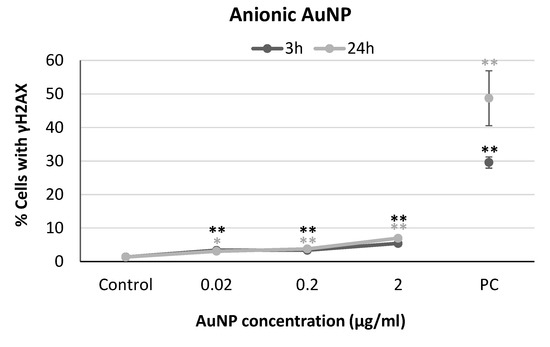
Figure 5.
Results from yH2AX analysis in SH-SY5Y cells exposed to anionic AuNPs for 3 and 24 h. PC: positive control (1 µg/mL BLM). * p < 0.05, ** p < 0.01, significant difference compared to the corresponding negative control.
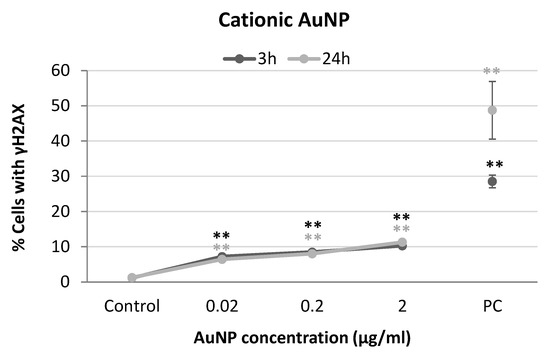
Figure 6.
Results from yH2AX analysis in SH-SY5Y cells exposed to cationic AuNPs for 3 and 24 h. PC: positive control (1 µg/mL BLM). ** p < 0.01, significant difference compared to the corresponding negative control.
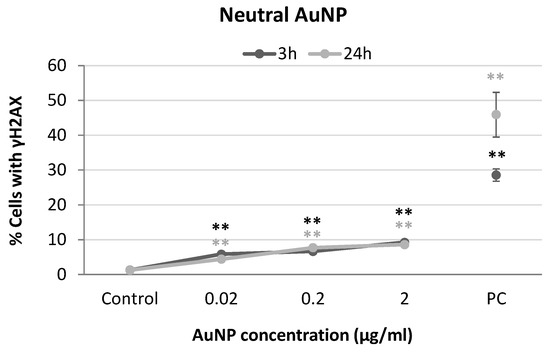
Figure 7.
Results from yH2AX analysis in SH-SY5Y cells exposed to neutral AuNPs for 3 and 24 h. PC: positive control (1 µg/mL BLM). ** p < 0.01, significant difference compared to the corresponding negative control.
Dose-dependent increases in the percentage of cells with γH2AX were observed in the neuronal cells treated with cationic AuNPs after both exposure times (3 h: r = 0.692; p < 0.01; 24 h: r = 0.900; p < 0.01) although the effects were more notable after 24 h (Figure 6).
Finally, significant increases in γH2AX levels were obtained for all conditions tested when SH-SY5Y cells were exposed to neutral AuNPs (Figure 7). Concentration-dependent relationships were also observed in this case for both exposure times (3 h: r = 0.824; p < 0.01; 24 h: r = 0.884; p < 0.01).
4. Conclusions
The results obtained from this work highlight the relevance of surface charge on AuNP toxicological behaviours. In particular, anionic and neutral AuNPs did not cause cytotoxic effects, while cationic nanoparticles showed cytotoxicity at the longest exposure time. Furthermore, cationic and neutral AuNPs showed only a moderate genotoxic potential after 24 h of exposure, while those with a negative charge did not induce a remarkable amount of double-strand breaks in DNA under any condition tested.
Author Contributions
Conceptualization, V.V., N.F.-B. and B.L.; methodology, A.T., S.B., M.P. and N.F.-B.; formal analysis, A.C., J.M. (Jesús Mosquera) and B.L.; writing—original draft preparation, V.V. and E.P.; writing—review and editing, J.M. (Josefina Méndez), B.L. and A.C.; supervision, V.V., N.F.-B. and B.L.; funding acquisition, V.V., J.M., A.C. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish Ministry of Science and Innovation: MCIN/AEI/10.13039/501100011033 (grant PID2020-114908GA-I00), Xunta de Galicia (ED431B 2022/16), CICA-Disrupting Project 2021SEM-A1, and Ministry of Education, Culture, and Sport (BEAGAL18/00142 to V.V.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kulkarni, S.; Kumar, S.; Acharya, S. Gold Nanoparticles in Cancer Therapeutics and Diagnostics. Cureus 2022, 14, e30096. [Google Scholar] [CrossRef] [PubMed]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Saha, D.; Chadha, T.S.; Raman, B.; Biswas, P. Non-invasive aerosol delivery and transport of gold nanoparticles to the brain. Sci. Rep. 2017, 7, 44718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, T.; Huang, W.; Yu, Y.; Sun, T. Applications of Gold Nanoparticles in Brain Diseases across the Blood-Brain Barrier. Curr. Med. Chem. 2022, 29, 6063–6083. [Google Scholar] [PubMed]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, L.; Mettenbrink, E.M.; Deangelis, P.L.; Wilhelm, S. Nanoparticle Toxicology. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 269–289. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R.J. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Costa, C.; Sharma, V.; Kiliç, G.; Pásaro, E.; Teixeira, J.P.; Dhawan, A.; Laffon, B. Comparative study on effects of two different types of titanium dioxide nanoparticles on human neuronal cells. Food Chem. Toxicol. 2013, 57, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bertólez, N.; Costa, C.; Brandão, F.; Duarte, J.A.; Teixeira, J.P.; Pásaro, E.; Valdiglesias, V.; Laffon, B. Evaluation of cytotoxicity and genotoxicity induced by oleic acid-coated iron oxide nanoparticles in human astrocytes. Environ. Mol. Mutagen. 2019, 60, 816–829. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Brandão, F.; Bessa, M.J.; Costa, S.; Valdiglesias, V.; Kiliç, G.; Fernández-Bertólez, N.; Quaresma, P.; Pereira, E.; Pásaro, E.; et al. In vitro cytotoxicity of superparamagnetic iron oxide nanoparticles on neuronal and glial cells. Evaluation of nanoparticle interference with viability tests. J. Appl. Toxicol. 2016, 36, 361–372. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5; Biological Evaluation of Medical Devices. Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).