Decellularized Extracellular Matrix Polycaprolactone/Chitosan Composite Nanofibrous Scaffolds for Periodontal Tissue Engineering †
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Electrospun Nanofibrous Scaffolds
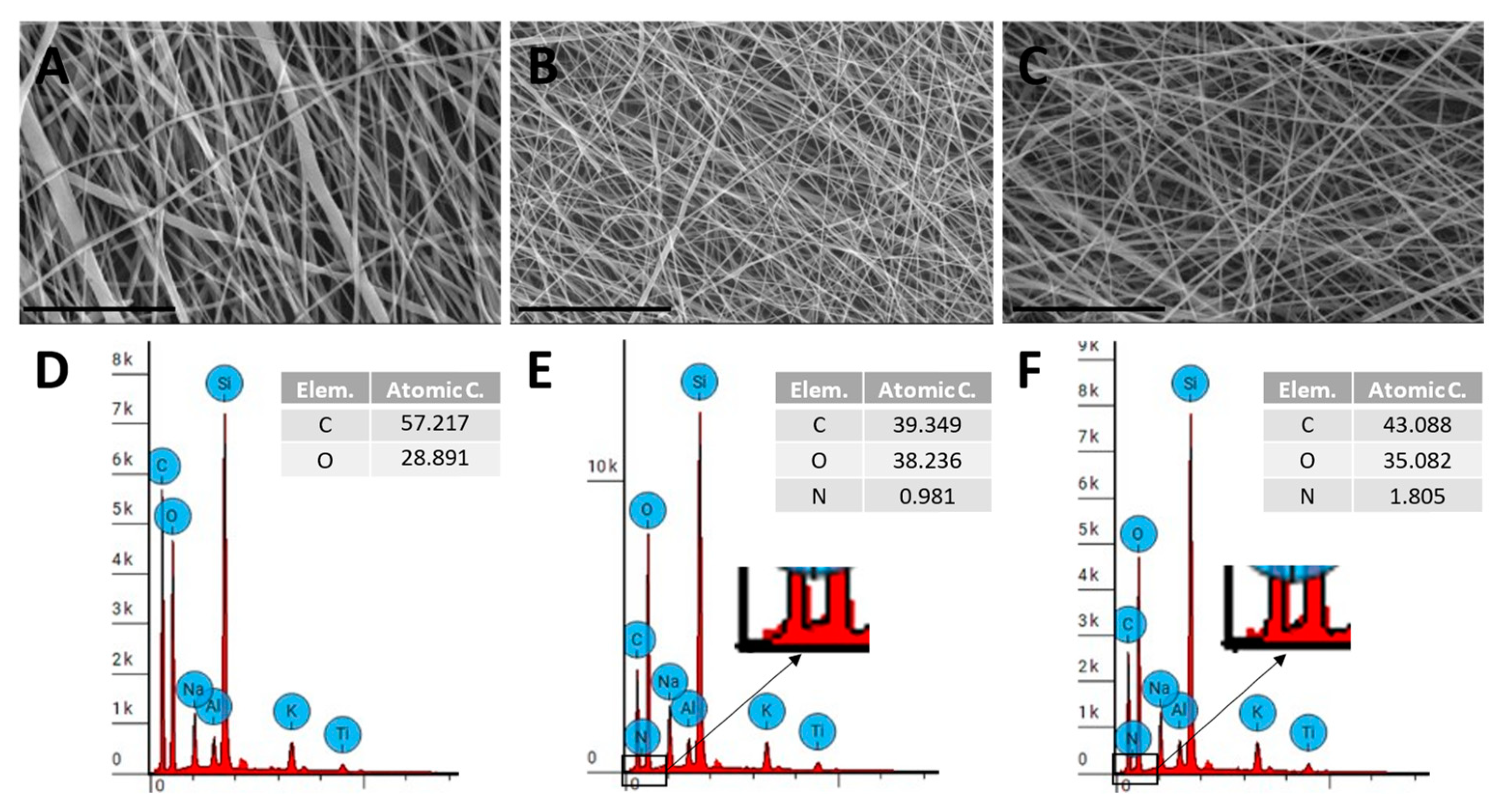
2.2. Characterization of Electrospun Scaffolds
2.3. In Vitro Cell Culture on Electrospun Scaffolds
2.3.1. Scaffold Preparation and PDLSC Seeding
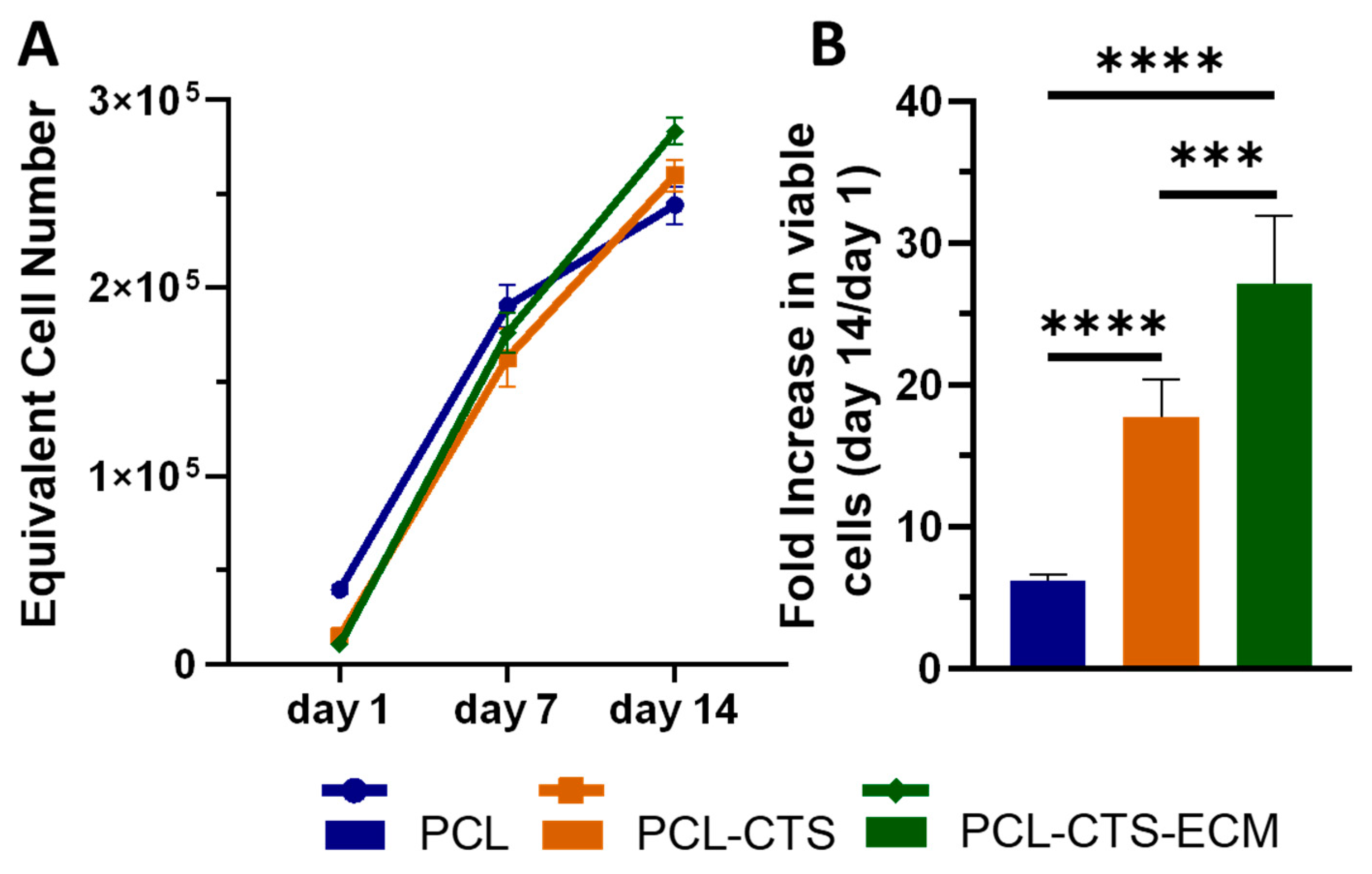
2.3.2. PDLSC Viability and Proliferation Assay
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Seymour, R.A.; Heasman, P.A. Current concepts in periodontal pathogenesis. Dent. Update 2004, 31, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Machado, V.; Leira, Y.; Proenca, L.; Chambrone, L.; Mendes, J.J. Economic burden of periodontitis in the United States and Europe: An updated estimation. J. Periodontol. 2022, 93, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Fan, L.; Alkildani, S.; Liu, L.; Emmert, S.; Najman, S.; Rimashevskiy, D.; Schnettler, R.; Jung, O.; Xiong, X.; et al. Barrier membranes for guided bone regeneration (gbr): A focus on recent advances in collagen membranes. Int. J. Mol. Sci. 2022, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Lin, K.; Yu, H. Advance of nano-composite electrospun fibers in periodontal regeneration. Front. Chem. 2019, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.; Silva, J.C.; Udangawa, R.N.; Cabral, J.M.; Ferreira, F.C.; da Silva, C.L.; Linhardt, R.J.; Vashishth, D. Co-culture cell-derived extracellular matrix loaded electrospun microfibrous scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 99, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Vaquette, C.; Theodoropoulos, C.; Hamlet, S.M.; Hutmacher, D.W.; Ivanovski, S. Decellularized periodontal ligament cell sheets with recellularization potential. J. Dent. Res. 2014, 93, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Hashimi, S.M.; Vaquette, C.; Bartold, P.M.; Hutmacher, D.W.; Ivanovski, S. The effect of decellularized tissue engineered constructs on periodontal regeneration. J. Clin. Periodontol. 2018, 45, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.; Ghebes, C.A.; Ahmed, M.; Kelder, C.; van Blitterswijk, C.A.; Saris, D.; Fernandes, H.A.M.; Moroni, L. Mesenchymal stromal cell-derived extracellular matrix influences gene expression of chondrocytes. Biofabrication 2013, 5, 025003. [Google Scholar] [CrossRef] [PubMed]
- de Jong, T.; Bakker, A.D.; Everts, V.; Smit, T.H. The intricate anatomy of the periodontal ligament and its development: Lessons for periodontal regeneration. J. Periodontal Res. 2017, 52, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Kao, H.H.; Kuo, C.Y.; Tagadur Govindaraju, D.; Chen, K.S.; Chen, J.P. Polycaprolactone/chitosan composite nanofiber membrane as a preferred scaffold for the culture of mesothelial cells and the repair of damaged mesothelium. Int. J. Mol. Sci. 2022, 23, 9517. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, W.; Tang, X.; Liu, X. Osteogenic induction of bone marrow mesenchymal cells on electrospun polycaprolactone/chitosan nanofibrous membrane. Dent. Mater. J. 2017, 36, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Qiao, F.; Chen, G.; Lv, Y. Demineralized and decellularized bone extracellular matrix-incorporated electrospun nanofibrous scaffold for bone regeneration. J. Mater. Chem. B 2021, 9, 6881–6894. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.S.; Cordeiro, R.; Moura, C.S.; da Silva, C.L.; Ferreira, F.C.; Silva, J.C.; Carvalho, M.S. Decellularized Extracellular Matrix Polycaprolactone/Chitosan Composite Nanofibrous Scaffolds for Periodontal Tissue Engineering. Mater. Proc. 2023, 14, 44. https://doi.org/10.3390/IOCN2023-14495
Santos MS, Cordeiro R, Moura CS, da Silva CL, Ferreira FC, Silva JC, Carvalho MS. Decellularized Extracellular Matrix Polycaprolactone/Chitosan Composite Nanofibrous Scaffolds for Periodontal Tissue Engineering. Materials Proceedings. 2023; 14(1):44. https://doi.org/10.3390/IOCN2023-14495
Chicago/Turabian StyleSantos, Mafalda S., Rachel Cordeiro, Carla S. Moura, Cláudia L. da Silva, Frederico Castelo Ferreira, João C. Silva, and Marta S. Carvalho. 2023. "Decellularized Extracellular Matrix Polycaprolactone/Chitosan Composite Nanofibrous Scaffolds for Periodontal Tissue Engineering" Materials Proceedings 14, no. 1: 44. https://doi.org/10.3390/IOCN2023-14495
APA StyleSantos, M. S., Cordeiro, R., Moura, C. S., da Silva, C. L., Ferreira, F. C., Silva, J. C., & Carvalho, M. S. (2023). Decellularized Extracellular Matrix Polycaprolactone/Chitosan Composite Nanofibrous Scaffolds for Periodontal Tissue Engineering. Materials Proceedings, 14(1), 44. https://doi.org/10.3390/IOCN2023-14495







