Study of Phosphine Tellurides as Precursors in the Synthesis of HgTe CQDs for IR Applications †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Tellurium Precursors
2.2.2. Characterization of Tellurium Precursors
2.2.3. Synthesis of Mercury Telluride Colloidal Quantum Dots
2.2.4. Characterization of HgTe CQDs
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keuleyan, S.E.; Guyot-Sionnest, P.; Delerue, C.; Allan, G. Mercury Telluride Colloidal Quantum Dots: Electronic Structure, Size-Dependent Spectra, and Photocurrent Detection up to 12 μm. ACS Nano 2014, 8, 8676–8682. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, T.; Im, S.H.; Seok, S.I.; Kim, K.W.; Kim, S.; Kim, S.-W. Bandgap engineered monodisperse and stable mercury telluride quantum dots and their application for near-infrared photodetection. J. Mater. Chem. 2011, 21, 15232–15236. [Google Scholar] [CrossRef]
- Kagan, C.R.; Lifshitz, E.; Sargent, E.H.; Talapin, D.V. Building devices from colloidal quantum dots. Science 2016, 353, aac5523. [Google Scholar] [CrossRef] [PubMed]
- Keuleyan, S.; Kohler, J.; Guyot-Sionnest, P. Photoluminescence of Mid-Infrared HgTe Colloidal Quantum Dots. J. Phys. Chem. C 2014, 118, 2749–2753. [Google Scholar] [CrossRef]
- Shuklov, I.A.; Mikhel, I.S.; Nevidimov, A.V.; Birin, K.P.; Dubrovina, N.V.; Lizunova, A.A.; Razumov, V.F. Mechanistic Insights into the Synthesis of Telluride Colloidal Quantum Dots with Trioctylphosphine-Tellurium. ChemistrySelect 2020, 5, 11896–11900. [Google Scholar] [CrossRef]
- Sun, H.; Wang, F.; Buhro, W.E. Tellurium Precursor for Nanocrystal Synthesis: Tris(dimethylamino)phosphine Telluride. ACS Nano 2018, 12, 12393–12400. [Google Scholar] [CrossRef] [PubMed]
- Goubet, N.; Jagtap, A.; Livache, C.; Martinez, B.; Portales, H.; Xu, X.Z.; Lobo, R.; Dubertret, B.; Lhuillier, E. Terahertz HgTe Nanocrystals: Beyond Confinement. J. Am. Chem. Soc. 2018, 140, 5033–5036. [Google Scholar] [CrossRef] [PubMed]
- Livache, C.; Martinez, B.; Goubet, N.; Greboval, C.; Qu, J.; Chu, A.; Royer, S.; Ithurria, S.; Silly, M.G.; Dubertret, B.; et al. A colloidal quantum dot infrared photodetector and its use for intraband detection. Nat. Commun. 2019, 10, 2125. [Google Scholar] [CrossRef] [PubMed]
- Chauzov, V.A.; Kostina, L.P. Alkylation In Situ of Arylphosphines Formed during Thermolysis of Hydrophosphoryl Compounds. J. Gen. Chem. USSR 1991, 61, 2181–2186. [Google Scholar]
- Du Mont, W.W.; Kroth, H.J. Zur Reaktion von Organophosphien mit Chalkogenen und Halogenen Rasche Ubertragung von Tellurund Jod Zwischen Phosphinen. J. Organomet. Chem. 1976, 113, C35–C37. [Google Scholar] [CrossRef]
- Chu, A.; Martinez, B.; Ferre, S.; Noguier, V.; Greboval, C.; Livache, C.; Qu, J.; Prado, Y.; Casaretto, N.; Goubet, N.; et al. HgTe Nanocrystals for SWIR Detection and Their Integration up to the Focal Plane Array. ACS Appl. Mater. Interfaces 2019, 11, 33116–33123. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Chen, M.; Guyot-Sionnest, P. Synthesis of Nonaggregating HgTe Colloidal Quantum Dots and the Emergence of Air-Stable n-Doping. J. Phys. Chem. Lett. 2017, 8, 2224–2228. [Google Scholar] [CrossRef] [PubMed]
- Brichkin, S.B.; Razumov, V.F. Colloidal quantum dots: Synthesis, properties and applications. Russ. Chem. Rev. 2016, 85, 1297–1312. [Google Scholar] [CrossRef]
- Im, S.H.; Kim, H.J.; Kim, S.W.; Kim, S.W.; Seok, S.I. Efficient HgTe colloidal quantum dot-sensitized near-infrared photovoltaic cells. Nanoscale 2012, 4, 1581–1584. [Google Scholar] [CrossRef] [PubMed]
- Nyquist, R.A. Interpreting Infrared, Raman, and Nuclear Magnetic Resonance Spectra; Academic Press: San Diego, CA, USA, 2001; Volume 2, pp. 65–83. [Google Scholar]
- Grazulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quiros, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An open-access collection of crystal structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef] [PubMed]
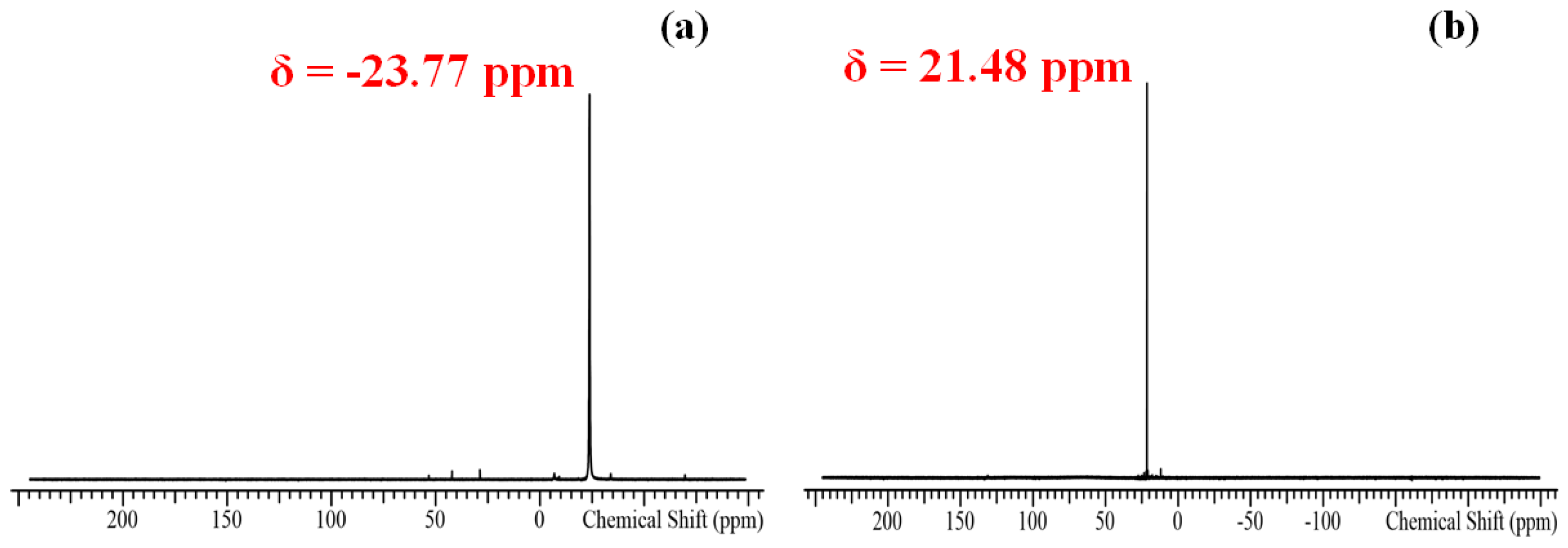


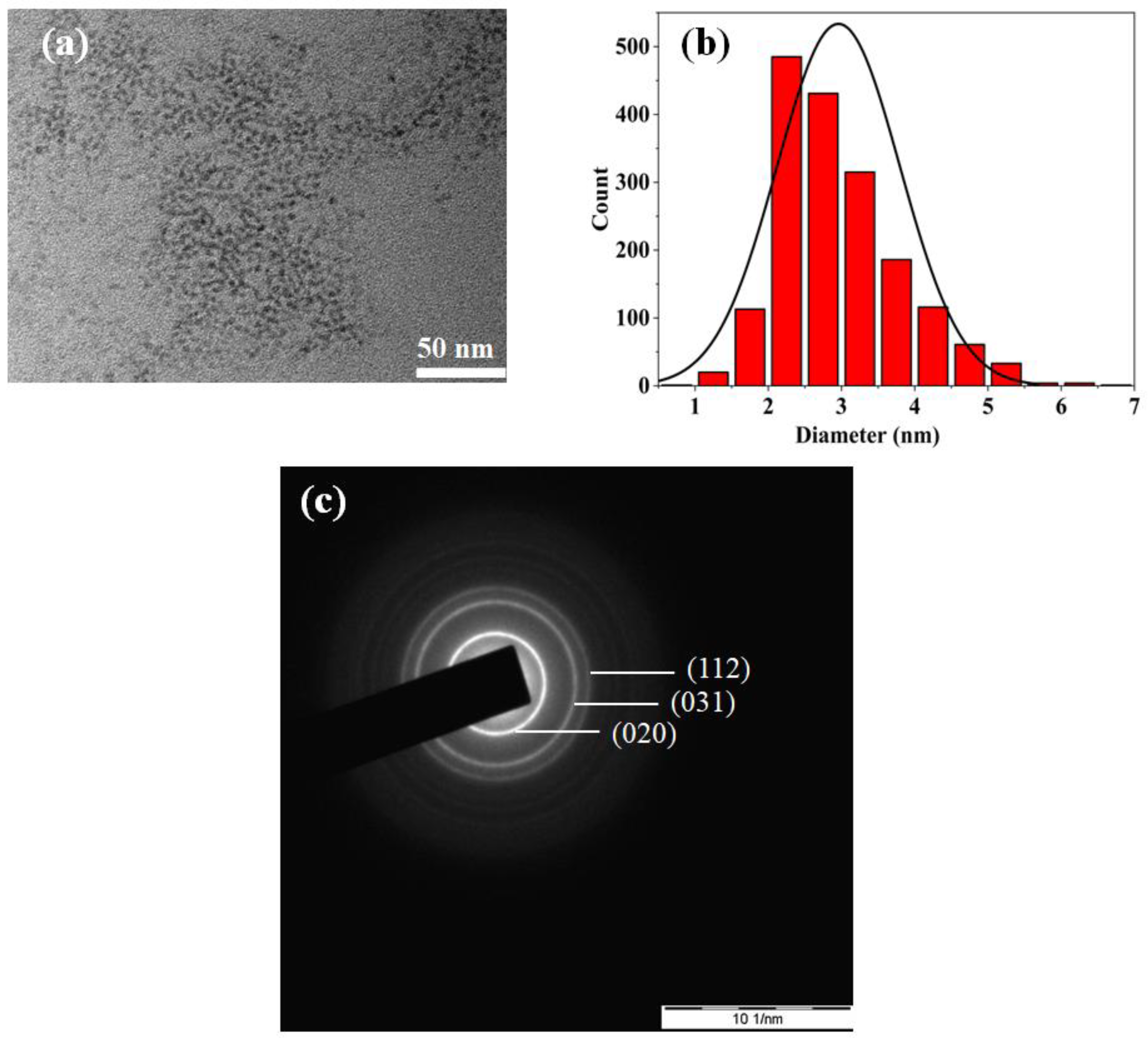
| Precursor | 15 min | FWHM | 60 min | FWHM | 90 min | FWHM |
|---|---|---|---|---|---|---|
| TDMAPTe/THF | 1297 | 214 | 1334 | 241 | 1342 | 253 |
| TOPTe/TOP | 1840 | 335 | 2052 | 375 | 2256 | 425 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mardini, A.A.; Shuklov, I.A.; Razumov, V.F. Study of Phosphine Tellurides as Precursors in the Synthesis of HgTe CQDs for IR Applications. Mater. Proc. 2023, 14, 40. https://doi.org/10.3390/IOCN2023-14512
Mardini AA, Shuklov IA, Razumov VF. Study of Phosphine Tellurides as Precursors in the Synthesis of HgTe CQDs for IR Applications. Materials Proceedings. 2023; 14(1):40. https://doi.org/10.3390/IOCN2023-14512
Chicago/Turabian StyleMardini, Alaa Alddin, Ivan Alekseevich Shuklov, and Vladimir Fedorovich Razumov. 2023. "Study of Phosphine Tellurides as Precursors in the Synthesis of HgTe CQDs for IR Applications" Materials Proceedings 14, no. 1: 40. https://doi.org/10.3390/IOCN2023-14512
APA StyleMardini, A. A., Shuklov, I. A., & Razumov, V. F. (2023). Study of Phosphine Tellurides as Precursors in the Synthesis of HgTe CQDs for IR Applications. Materials Proceedings, 14(1), 40. https://doi.org/10.3390/IOCN2023-14512







