Controlling the Response of Gas-Sensitive Zinc Oxide Nanostructures to Water Vapor †
Abstract
1. Introduction
2. Experiment
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panda, S.; Mehlawat, S.; Dhariwal, N.; Kumar, A.; Sanger, A. Comprehensive review on gas sensors: Unveiling recent developments and addressing challenges. Mater. Sci. Eng. 2024, 308, 117616. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc Oxide Nanostructures for NO2 Gas–Sensor Applications: A Review. Nano-Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials 2022, 12, 3066. [Google Scholar] [CrossRef]
- Franco, M.A.; Conti, P.P.; Andre, R.S.; Correa, D.S. A review on chemiresistive ZnO gas sensors. Sens. Act. Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, G.; Umar, A. Zinc oxide nanomaterials for photocatalytic degradation of methyl orange: A review. Nanosci. Nanotechnol. Lett. 2014, 6, 631–650. [Google Scholar] [CrossRef]
- Gerbreders, V.; Krasovska, M.; Sledevskis, E.; Gerbreders, A.; Mihailova, I.; Tamanis, E.; Ogurcovs, A. Hydrothermal synthesis of ZnO nanostructures with controllable morphology change. Cryst. Eng. Comm. 2020, 22, 1346–1358. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y. Recent progress on anti-humidity strategies of chemiresistive gas sensors. Materials 2022, 15, 8728. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of temperature and humidity on the sensing performance of TiO2 nanowire-based ethanol vapor sensors. Nanotechnology 2021, 32, 325501. [Google Scholar] [CrossRef]
- Sun, K.; Zhan, G.; Zhang, L.; Wang, Z.; Lin, S. Highly sensitive NO2 gas sensor based on ZnO nanoarray modulated by oxygen vacancy with Ce doping. Sens. Act. B 2023, 379, 133294. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensors. Solid State Ion. 2021, 116, 115544. [Google Scholar] [CrossRef]
- Chang, C.M.; Hon, M.H.; Leu, I.C. Preparation of ZnO nanorod arrays with tailored defect-related characterisitcs and their effect on the ethanol gas sensing performance. Sens. Act. B 2010, 151, 15–20. [Google Scholar] [CrossRef]
- Bang, J.H.; Kwon, Y.J.; Lee, J.-H.; Mirzaei, A.; Lee, H.Y.; Choi, H.; Kim, S.S.; Jeong, Y.K.; Kim, H.W. Proton-beam engineered surface-point defects for highly sensitive and reliable NO2 sensing under humid environments. J. Hazard Mater. 2021, 416, 125841. [Google Scholar] [CrossRef] [PubMed]
- Struzzi, C.; Scardamaglia, M.; Casanova-Chafer, J.; Calavia, R.; Colomer, J.-F.; Kondyurin, A.; Bilek, M.; Britun, N.; Snyders, R. Exploiting sensor geometry for enhanced gas sensing properties of fluorinated carbon nanotubes under humid environment. Sens. Act. B 2019, 281, 945–952. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Zhang, M.; Wu, Z.; Kong, D.; Qian, H.; Sub, B. Etching process enhanced H2O2 sensing performance of SnO2/Zn2SnO4 with reliable anti-humidity ability. Anal. Methods 2022, 14, 3335–3344. [Google Scholar] [CrossRef]
- Nalimova, S.; Shomakhov, Z.; Bobkov, A.; Moshnikov, V. Sacrificial Doping as an Approach to Controlling the Energy Properties of Adsorption Sites in Gas-Sensitive ZnO Nanowires. Micro 2023, 3, 591–601. [Google Scholar] [CrossRef]
- Nalimova, S.S.; Moshnikov, V.A.; Myakin, S.V. Controlling surface functional composition and improving the gas-sensing properties of metal oxide sensors by electron beam processing. Glass Phys. Chem. 2016, 42, 597–601. [Google Scholar] [CrossRef]
- Bozhinova, A.S.; Kaneva, N.V.; Syuleiman, S.A.; Papazova, K.I.; Dimitrov, D.T.; Kononova, I.E.; Nalimova, S.S.; Moshnikov, V.A.; Terukov, E.I. Study of the photocatalytic and sensor properties of ZnO/SiO2 nanocomposite layers. Semiconductors 2013, 47, 1636–1640. [Google Scholar] [CrossRef]
- Chudinova, G.K.; Nagovitsyn, I.A.; Gadzhiev, T.T.; Danilov, V.V.; Kurilkin, V.V.; Moshnikov, V.A.; Nalimova, S.S.; Kononova, I.E. Fluorescence of ZnO:SiO2 and SnO2:SiO2 nanosized composite films under the action of human serum albumin. Dokl. Phys. Chem. 2014, 456, 74–76. [Google Scholar] [CrossRef]
- Nalimova, S.; Shomakhov, Z.; Moshnikov, V. Binary and Ternary Oxide Nanostructured Multisystems for Gas Sensors. Eng. Proc. 2023, 48, 47. [Google Scholar] [CrossRef]
- Shomakhov, Z.V.; Nalimova, S.S.; Bobkov, A.A.; Moshnikov, V.A. X-ray photoelectron spectroscopy of the surface layers of faceted zinc-oxide nanorods. Semiconductors 2022, 56, 450–454. [Google Scholar] [CrossRef]
- Nalimova, S.S.; Shomakhov, Z.V.; Gerasimova, K.V.; Punegova, K.N.; Guketlov, A.M.; Kalmykov, R.M. Gas-sensitive composite nanostructures based on zinc oxide for detecting organic solvent vapors. Phys. Chem. Asp. Study Clust. Nanostruct. Nanomater. 2023, 14, 678–687. [Google Scholar] [CrossRef]
- Nalimova, S.S.; Shomakhov, Z.V.; Kondratev, V.M.; Moshnikov, V.A.; Karmokov, A.M. Investigation of hierarchical gas-sensing ZnFe2O4 nanostructures. J. Surf. Inv. X-Ray Synchrotron Neutron Tech. 2023, 17, S416–S422. [Google Scholar] [CrossRef]
- Gulevich, D.; Rumyantseva, M.; Gerasimov, E.; Marikutsa, A.; Krivetskiy, V.; Shatalova, T.; Khmelevsky, N.; Gaskov, A. Nanocomposites SnO2/SiO2 for CO Gas Sensors: Microstructure and Reactivity in the Interaction with the Gas Phase. Materials 2019, 12, 1096. [Google Scholar] [CrossRef]
- Saruhan, B.; Lontio Fomekong, R.; Nahirniak, S. Influences of semiconductor metal oxide properties on gas sensing characteristics. Front. Sens. 2021, 2, 657931. [Google Scholar] [CrossRef]
- Jin, X.; Zha, L.; Wang, F.; Wang, Y.; Zhang, X. Fully integrated wearable humidity sensor for respiration monitoring. Front. Bioeng. Biotechnol. 2022, 10, 1070855. [Google Scholar] [CrossRef]
- Tsai, F.S.; Wang, S.J. Enhanced sensing performance of relative humidity sensors using laterally grown ZnO nanosheets. Sens. Act. B 2014, 193, 280–287. [Google Scholar] [CrossRef]
- Tai, W.P.; Oh, J.H. Humidity sensing behaviors of nanocrystalline Al-doped ZnO thin films prepared by sol–gel process. J. Mater. Sci. Mater. Electron. 2002, 13, 391–394. [Google Scholar] [CrossRef]
- Agmon, N. The grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
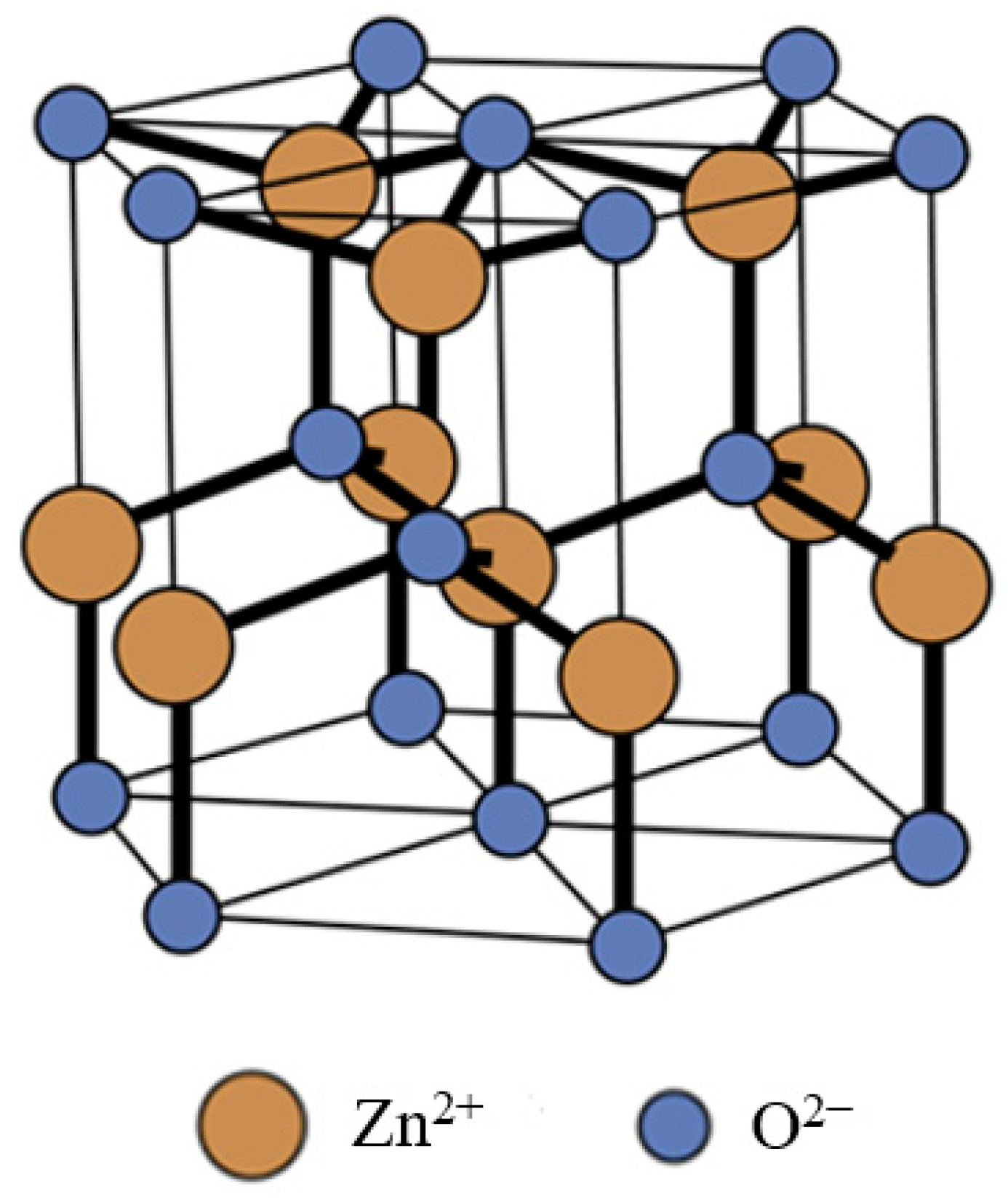
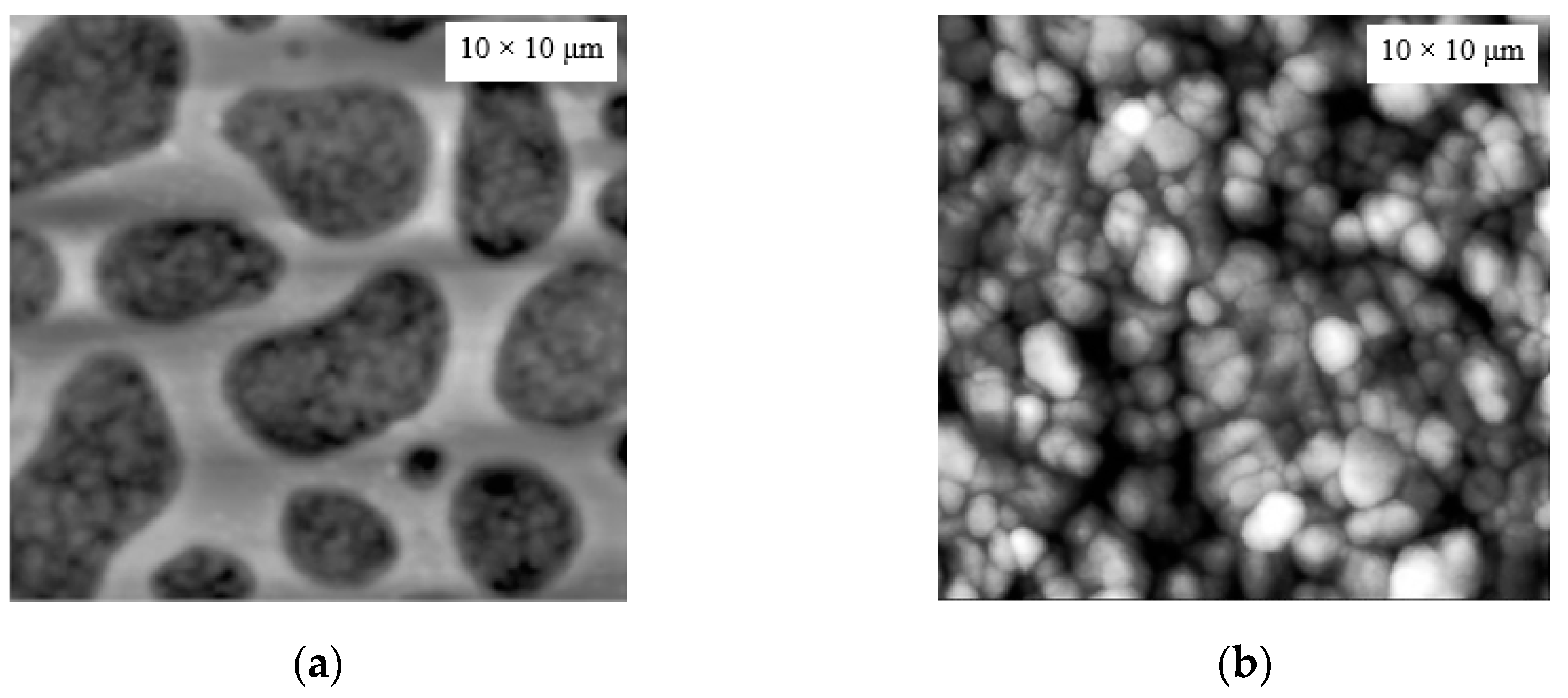
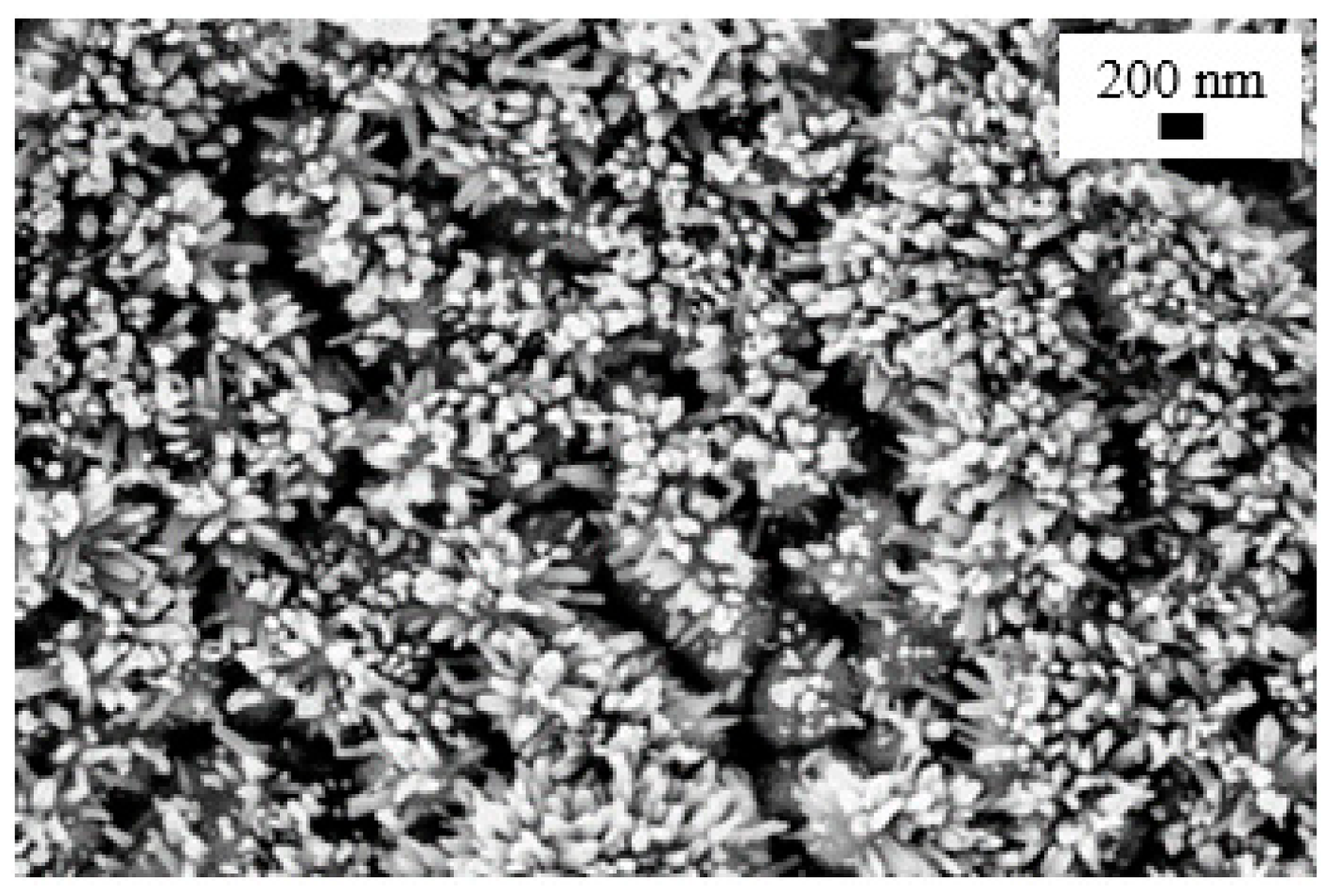
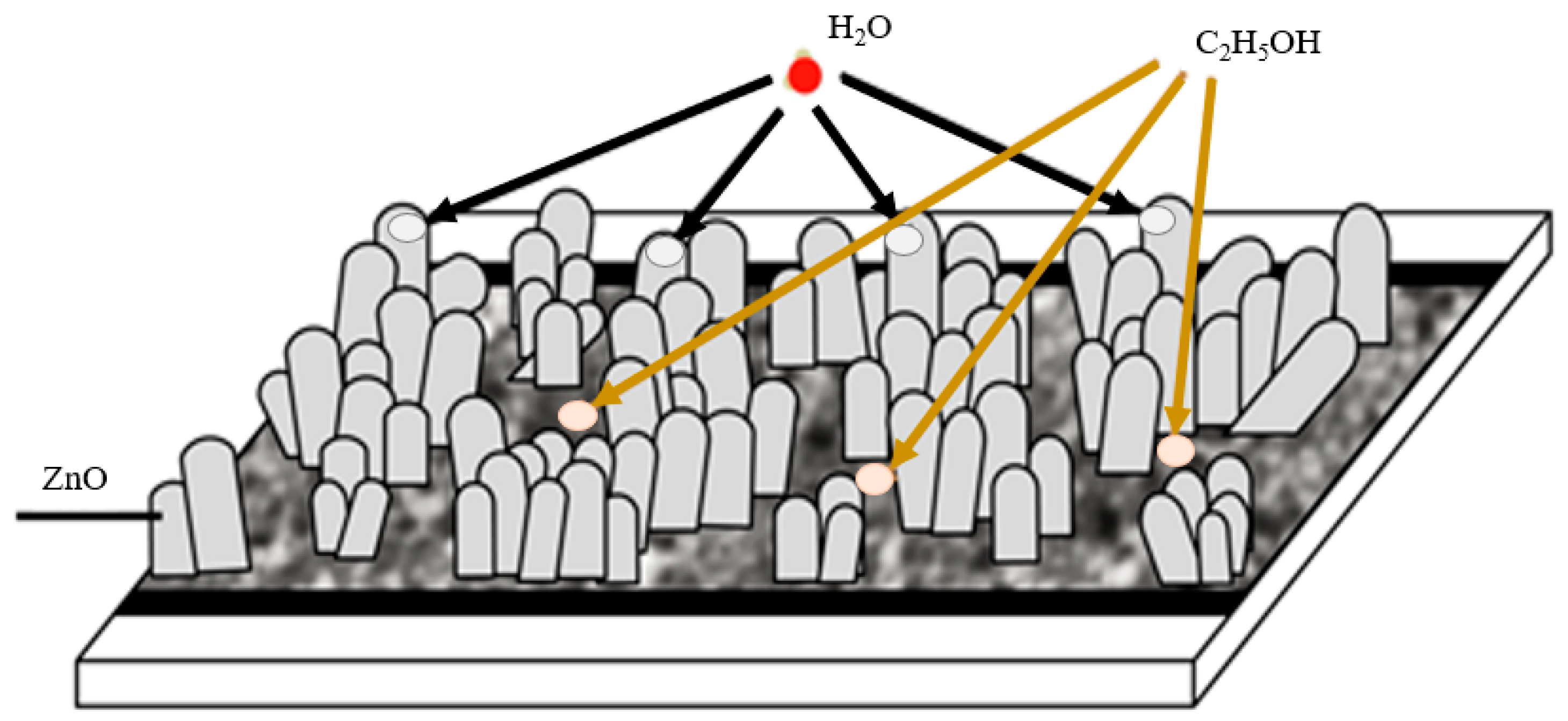
| Seed Layer | Nanowires Type | S(C3H7OH) | S(H2O) |
|---|---|---|---|
| ZnO-SiO2 nanocomposites | ZnO(I) | 1.58 | 1.84 |
| ZnO(Br) | 1.67 | 2.17 | |
| ZnO nanoparticles | ZnO(I) | 2.97 | 1.21 |
| ZnO(Br) | 4.03 | 1.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nalimova, S.S.; Shomakhov, Z.V.; Miroshkina, V.V.; Bui, C.D.; Moshnikov, V.A. Controlling the Response of Gas-Sensitive Zinc Oxide Nanostructures to Water Vapor. Eng. Proc. 2024, 82, 98. https://doi.org/10.3390/ecsa-11-20477
Nalimova SS, Shomakhov ZV, Miroshkina VV, Bui CD, Moshnikov VA. Controlling the Response of Gas-Sensitive Zinc Oxide Nanostructures to Water Vapor. Engineering Proceedings. 2024; 82(1):98. https://doi.org/10.3390/ecsa-11-20477
Chicago/Turabian StyleNalimova, Svetlana S., Zamir V. Shomakhov, Vlada V. Miroshkina, Cong D. Bui, and Vyacheslav A. Moshnikov. 2024. "Controlling the Response of Gas-Sensitive Zinc Oxide Nanostructures to Water Vapor" Engineering Proceedings 82, no. 1: 98. https://doi.org/10.3390/ecsa-11-20477
APA StyleNalimova, S. S., Shomakhov, Z. V., Miroshkina, V. V., Bui, C. D., & Moshnikov, V. A. (2024). Controlling the Response of Gas-Sensitive Zinc Oxide Nanostructures to Water Vapor. Engineering Proceedings, 82(1), 98. https://doi.org/10.3390/ecsa-11-20477






