Abstract
Medicinal plants contain a wide range of bioactive compounds including antioxidants. Thus, the evaluation of the antioxidant capacity of medicinal plant extracts used in phytotherapy is of practical interest. Water extracts from 11 plants obtained by sonication for 30 min were studied by cyclic voltammetry at bare glassy carbon electrode (GCE) and GCE modified with a mixture of 1 mg mL−1 CeO2 and SnO2 nanoparticles (NPs) dispersed in 0.10 mM cetylpyridinium bromide. A two-step chronoamperometric approach (at 400 and 900 mV for 75 s each one) was developed to estimate the antioxidant capacity of medicinal plant extracts. A strong and very strong correlation level was obtained between the antioxidant capacity and total phenolic contents or antioxidant capacity toward 2,2-diphenyl-1-picrylhydrazyl (DPPH•).
1. Introduction
Since ancient times, medicinal plants have been used for the treatment of human diseases. The wide range of bioactive compounds contained in plants stipulates the development of new phytopharmaceuticals [1]. Traditional phytotherapy is also still applied as part of complex treatment [2,3].
Antioxidants are one of the largest groups of bioactive compounds of plant origin [4] that are widely distributed in medicinal plants. Thus, the evaluation of the antioxidant capacity of medicinal plant extracts used in phytotherapy is of practical interest.
Electrochemical methods have been shown to be a highly effective tool for the evaluation of total antioxidant parameters of plant materials [5]. They combine high precision, cost-efficiency, rapid response, simplicity, and in-field applicability. In application to medicinal plants, the electrochemical response usually corresponds to the contents of several antioxidants of similar structure. Depending on the method applied, the impact of major components can be evaluated as it occurs in voltammetry or the total contents of oxidizable components can be obtained if chrono methods are used. The last are preferable because they allow estimation of a wider range of components, including those present at low concentrations [6,7].
Thus, the current work focused on the development of a novel chronoamperometric method using a glassy carbon electrode (GCE) modified with a mixture of 1 mg mL−1 CeO2 and SnO2 nanoparticles (NPs) dispersed in 0.10 mM cetylpyridinium bromide for the determination of the antioxidant capacity of water extracts from medicinal plants obtained by sonication.
2. Materials and Methods
Commercially available medicinal plant material (11 samples) was studied. The corresponding water extracts were obtained by sonication for 30 min in an ultrasonic bath (WiseClean WUC-A03H) (DAIHAN Scientific Co., Ltd., Wonju-si, Republic of Korea). The plant:water ratio was 1:10, 1:20, or 1:33 depending on the type of plant material, its water absorption and the consumption coefficients. The extracts were filtered, their volume was adjusted to the initial value, and they were used in the subsequent studies.
Gallic acid (99% purity) and Folin-Ciocalteu reagent were obtained from Sigma (Steinheim, Germany) and 2,2-diphenyl-1-picrylhydrazyl (DPPH•) was purchased from Aldrich (Steinheim, Germany). Standard solutions of gallic acid (100 mg mL−1) and DPPH• (0.20 mM) were prepared by dissolving the exact weight of the substance in distilled water and methanol (c.p.), respectively. The Folin-Ciocalteu reagent solution was diluted in a 1:10 ratio with distilled water prior to the experiment.
CeO2 and SnO2 NPs were used as electrode surface modifiers. Their 1 mg mL−1 mixture was prepared from commercial reagents (10% CeO2 NPs water dispersion from Sigma–Aldrich (St. Louis, MO, USA) and SnO2 NPs powder from Aldrich (Steinheim, Germany)) using a 0.10 mM solution of cetylpyridinium bromide as the dispersive medium. The standard 1.0 mM solution of cetylpyridinium bromide in water was prepared from a 98% purity reagent (Aldrich, Steinheim, Germany).
Electrode surface modification was performed by drop casting 5 μL of dispersion of CeO2 and SnO2 NPs mixture after careful cleaning of the GCE surface with alumina slurry (0.05 μm grain).
Voltammetric and chronoamperometric measurements were carried out with a potentiostat/galvanostat μAutolab Type III (Eco Chemie B.V., Utrecht, The Netherlands) using GPES 4.9 software. GCE (3 mm diameter) from CH Instruments, Inc. (Bee Cave, TX, USA) or a modified electrode, reference Ag/AgCl electrode, and auxiliary electrode (platinum wire) were placed in an electrochemical glass cell containing phosphate buffer pH 7.0 and cyclic voltammograms were recorded from 0.0 to 1.2 V with a potential scan rate of 100 mV s−1. Chronoamperometry was performed at +400 and +900 mV in phosphate buffer pH 7.0.
The total phenolic content was measured by a standard spectrophotometric method based on the reaction with the Folin-Ciocalteu reagent [8]. Gallic acid was used as a standard. The total phenolic content was recalculated per 100 mL of the extract.
The antioxidant activity toward DPPH• was studied by spectrophotometry [9] and expressed as a relative percentage of the decrease in DPPH• absorption after the reaction with the antioxidants of the extract. The extract volume was 50 μL for all samples under study.
3. Results and Discussion
3.1. Voltammetric Behavior of Water Extracts from Medicinal Plants
At first, the cyclic voltammetry on bare GCE in phosphate buffer pH 7.0 was applied for the characterization of medicinal plant water extracts. All samples excluding Urticae folia showed oxidation steps, the shapes and potentials of which depended on the type of medicinal plant (Figure 1). The oxidation potentials varied due to the classes of compounds contained in each sample (Table 1). The contents of oxidizable compounds also affect the oxidation potential value as far as overlap of the oxidation steps occur in the case of multicomponent samples that lead to the shift of the oxidation potential value. However, a high sample volume should be used for the majority of extracts to obtain a more or less pronounced signal on the voltammograms. Nevertheless, the oxidation currents at the nanoampere level are observed for several samples or even in the full absence of the oxidation steps as for the Urticae folia extract (Table 1).
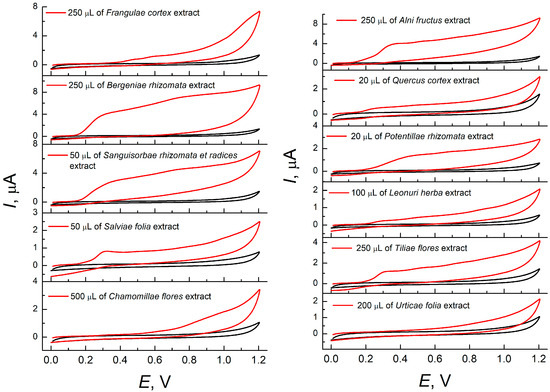
Figure 1.
Cyclic voltammograms of medicinal plant water extracts on bare GCE in phosphate buffer pH 7.0. The potential scan rate is 100 mV s−1.

Table 1.
Voltammetric characteristics of water extracts from medicinal plants on the bare GCE and the GCE modified with a mixture of CeO2 and SnO2 NPs in phosphate buffer pH 7.0 (n = 5; p = 0.95).
Phenolic compounds are one of the most widely distributed groups of bioactive compounds in medicinal plants [10,11,12] that are electroactive at anodic potentials. As shown earlier on typical plant phenolics [13,14,15], the application of chemically modified electrodes based on CeO2 or SnO2 NPs improves the voltammetric response parameters. Therefore, GCE modified with a mixture of CeO2 and SnO2 NPs dispersed in 0.10 mM cetylpyridinium bromide was tested for the sensing of water extracts from medicinal plants.
There are well-defined oxidation steps on the voltammograms from the modified electrode. The oxidation potentials are close to those observed at the bare GCE (Table 1). The exception is Potentillae rhizomata extract, for which a 110 mV shift of oxidation potential to less positive values was registered. Changes in the voltammetric characteristics were also obtained for the Quercus cortex extract. Two oxidation steps were shifted close to each other, forming one oxidation peak. The oxidation currents of the extracts at the modified electrode were increased vs. bare GCE, which is explained by a 4.9-fold increase in the electroactive surface area of the modified electrode (40 ± 2 and 8.2 ± 0.3 mm2, respectively).
3.2. Chronoamperometry of Water Extracts from Medicinal Plants
As known [5], the voltammetric response of medicinal plant extracts is integral, i.e., each oxidation step is caused by the presence of various antioxidants, including those that are structurally related. Moreover, the major contributors to the voltammetric response are components with a high content in the sample. Chronoamperometry allows for covering a wider range of antioxidants independently of their contents, providing more information about the sample and improvements in the accuracy of the antioxidant capacity assay.
For all extracts under study, the first oxidation step occurred in the range of 310–400 mV and the second one in the range of 520–900 mV at the modified electrode (Table 1). These values agree well with the classification of the antioxidants by their reducing power [16,17].
Therefore, two-step chronoamperometry was used at 400 and 900 mV for antioxidant capacity evaluation. Components with a low concentration also had an impact in the analytical response in this case. The effect of the electrolysis time on the chronoamperometric response of medicinal plant extracts was studied. The electrolysis steady state was achieved after 75 s in each step. The corresponding chronoamperograms are presented in Figure 2.
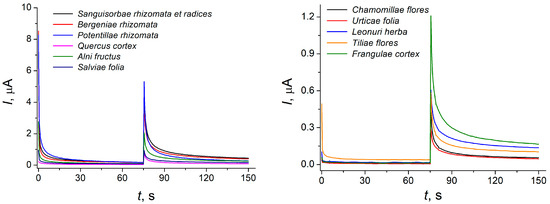
Figure 2.
Two-step background subtracted chronoamperograms of 50 μL of medicinal plant extracts at the GCE modified with a mixture of 1 mg mL−1 CeO2 and SnO2 NPs. The supporting electrolyte is phosphate buffer pH 7.0, E1 = 400 mV, E2 = 900 mV.
The antioxidant capacity has been expressed as current at the corresponding potential, recalculated per 100 mL of the extract (Table 2).

Table 2.
Antioxidant capacity (AOC) of water extracts from medicinal plants based on chronoamperometric measurements (n = 5; p = 0.95).
The antioxidant capacity at 900 mV reflects the total contents of the antioxidants, i.e., the components of high, intermediate, and low antioxidant power [16]. The highest antioxidant capacity was observed for high-density types of plant material in particular roots, rhizomes, and bark that agrees well with the literature data [18,19,20]. The lower antioxidant capacity was obtained for the Urticae folia and Chamomillae flores, being in line with the voltammetric data.
Comparison of the data obtained with the standard antioxidant parameters (total phenolic contents and antioxidant capacity toward DPPH•) showed a strong (r = 0.7–0.9) and very strong (r = 0.9–1.0) correlation level (Table 3) according to the Chaddock scale.

Table 3.
Correlation of antioxidant parameters of water extracts from medicinal plants obtained by chronoamperometry and spectrophotometry (n = 11; p = 0.95), rcrit = 0.6021.
The results obtained confirm the accuracy of the developed chronoamperometric method. Furthermore, the methos developed is simple and does not require the application of additional specific reagents such as the Folin-Ciocalteu reagent or DPPH•, which is unstable and highly affected by the presence of light and water. The use of phosphate buffer pH 7.0 is close to physiological conditions, making possible the partial prediction of medicinal plants’ affects in biosystems. Another advantage of chronoamperometry is a rapid response due to the absence of an incubation stage that makes the method applicable for fast screening tests.
4. Conclusions
A two-step chronoamperometric method has been developed for the evaluation of the antioxidant capacity of water extracts from medicinal plants for the first time. GCE modified with the mixture of CeO2 and SnO2 NPs dispersed in cetylpyridinium bromide provides improvement of the sample antioxidant response and enlarges the number of components contributing to the antioxidant parameters. The use of two potentials for the electrolysis allows discrimination of the antioxidants by power. The chronoamperometric approach is based on the direct response of antioxidants and does not need the special reagents usually used for the evaluation of the antioxidant properties of plant samples.
Further development in the field can be focused on the fabrication of portable devices for the on site monitoring of antioxidants in samples of plant origin. To solve this problem, electrodes with a long-term stable and reliable response have to be developed.
Author Contributions
Conceptualization, G.Z.; methodology, G.Z.; validation, Y.L. and G.Z.; investigation, Y.L.; writing—original draft preparation, G.Z.; writing—review and editing, G.Z.; visualization, Y.L. and G.Z.; supervision, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tewari, A.; Tiwari, S. (Eds.) Synthesis of Medicinal Agents from Plants; Elsevier: Amsterdam, The Netherlands, 2018; 357p. [Google Scholar]
- Capasso, F.; Gaginella, T.S.; Grandolini, G.; Izzo, A.A. Phytotherapy: A Quick Reference to Herbal Medicine; Springer: Berlin/Heidelberg, Germany, 2003; 424p. [Google Scholar] [CrossRef]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Kalmykova, A. Electrochemical characterization of the antioxidant properties of medicinal plants and products: A review. Molecules 2023, 28, 2308. [Google Scholar] [CrossRef] [PubMed]
- Kalmykova, A.; Ziyatdinova, G. Screening of essential oil antioxidant capacity using electrode modified with carboxylated multi-walled carbon nanotubes. Eng. Proc. 2022, 27, 48. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Kozlova, E.; Morozova, E.; Budnikov, H. Chronocoulometric method for the evaluation of antioxidant capacity of medicinal plant tinctures. Anal. Methods 2018, 10, 4995–5003. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.-T.; Gan, R.-Y.; Zhang, Y.; Xu, X.-R.; Xia, E.-Q.; Li, H.-B. Total phenolic contents and antioxidant capacities of herbal and tea infusions. Int. J. Mol. Sci. 2011, 12, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Evans, W.C. Trease and Evans’ Pharmacognosy, 16th ed.; Saunders Ltd.: London, UK, 2009; 600p. [Google Scholar]
- Akhtar, N.; Ihsan-ul-Haq; Mirza, B. Phytochemical analysis and comprehensive evaluation of antimicrobial and antioxidant properties of 61 medicinal plant species. Arab. J. Chem. 2018, 11, 1223–1235. [Google Scholar] [CrossRef]
- Pullaiah, T. (Ed.) Phytochemical Composition and Pharmacy of Medicinal Plants; CRC Press: New York, NY, USA, 2023; 1292p. [Google Scholar]
- Ziyatdinova, G.; Ziganshina, E.; Romashkina, S.; Budnikov, H. Highly sensitive amperometric sensor for eugenol quantification based on CeO2 nanoparticles and surfactants. Electroanalysis 2017, 29, 1197–1204. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, G.; Li, M.; Wang, C.; Fang, B. Determination of rutin using a CeO2 nanoparticle-modified electrode. Microchim. Acta 2007, 158, 269–274. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Yakupova, E.; Davletshin, R. Voltammetric determination of hesperidin on the electrode modified with SnO2 nanoparticles and surfactants. Electroanalysis 2021, 33, 2417–2427. [Google Scholar] [CrossRef]
- Blasco, A.J.; Rogerio, M.C.; González, M.C.; Escarpa, A. “Electrochemical Index” as a screening method to determine “total polyphenolics” in foods: A proposal. Anal. Chim. Acta 2005, 539, 237–244. [Google Scholar] [CrossRef]
- Haque, M.A.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Electrochemical methods to evaluate the antioxidant activity and capacity of foods: A review. Electroanalysis 2021, 33, 1419–1435. [Google Scholar] [CrossRef]
- Lone, A.S.; Shahnawaz, M.; Singh, N.; Pervez, S.; Ravindran, K.C. Metabolomic and antioxidant potential analyses of the rhizome and leaves of Podophyllum hexandrum Royle: A comparative account. Biocatal. Agric. Biotechnol. 2023, 52, 102836. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.; Zhang, T.; Qiao, Q.; Hou, X. Phenolic profiles and antioxidant activity in different organs of Sinopodophyllum hexandrum. Front. Plant Sci. 2022, 13, 1037582. [Google Scholar] [CrossRef] [PubMed]
- Krupanidhi, A.M.; Prakash, D.; Anusha, M.M.; Deepika, B.V.; Sameera, H.R.; Srinivas, G.; Soundarya, R. Antioxidant properties of medicinal plants: A review. Sys. Rev. Pharm. 2022, 13, 457–463. Available online: https://www.sysrevpharm.org/articles/antioxidant-properties-of-medicinal-plants-a-review.pdf (accessed on 29 July 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).