Electropolymerized Dyes as Sensing Layer for Natural Phenolic Antioxidants of Essential Oils †
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
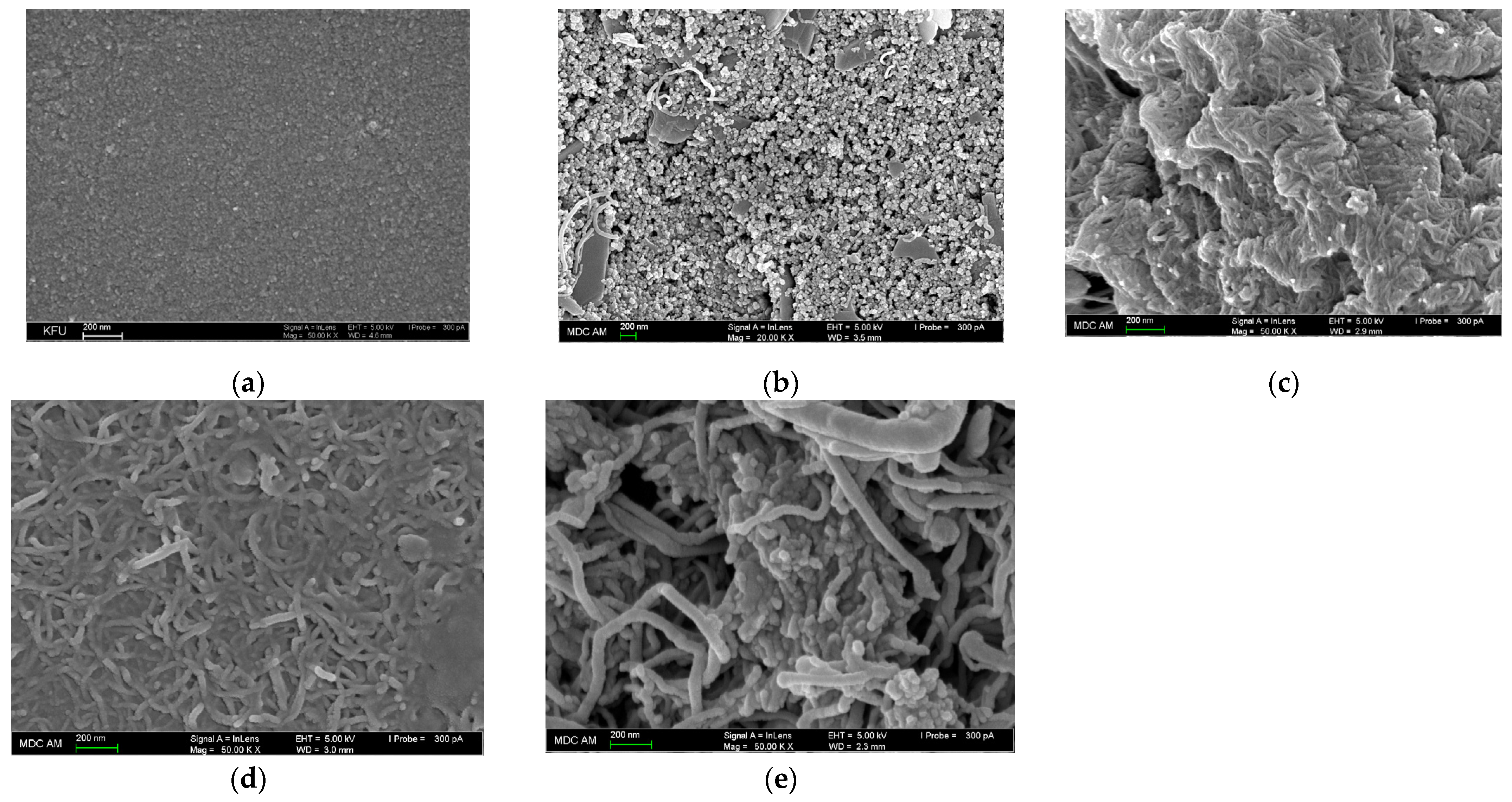
3.1. Polymer-Based Sensors Fabrication and Charcaterization
3.2. Sensing of Phenolic Antioxidants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Ni, Z.-J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Zuzarte, M.; Salgueiro, L. Essential oils chemistry. In Bioactive Essential Oils and Cancer; de Sousa, D.P., Ed.; Springer: Cham, Switzerland, 2015; pp. 19–61. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Budnikov, H.C. Natural phenolic antioxidants in bioanalytical chemistry: State of the art and prospects of development. Russ. Chem. Rev. 2015, 84, 194–224. [Google Scholar] [CrossRef]
- Bertuola, M.; Fagali, N.; de Mele, M.F.L. Detection of carvacrol in essential oils by electrochemical polymerization. Heliyon 2020, 6, e03714. [Google Scholar] [CrossRef] [PubMed]
- Robledo, S.N.; Pierini, G.D.; Nieto, C.H.D.; Fernández, H.; Zon, M.A. Development of an electrochemical method to determine phenolic monoterpenes in essential oils. Talanta 2019, 196, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Song, B. Electrochemical sensing of monoterpene phenols in plant essential oils using a molecularly imprinted polymer dual-template sensor. Int. J. Electrochem. Sci. 2023, 18, 100381. [Google Scholar] [CrossRef]
- Pierini, G.D.; Bortolato, S.A.; Robledo, S.N.; Alcaraz, M.R.; Fernández, H.; Goicoechea, H.C.; Zon, M.A. Second-order electrochemical data generation to quantify carvacrol in oregano essential oils. Food Chem. 2022, 368, 130840. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Ziganshina, E.; Budnikov, H. Voltammetric sensing and quantification of eugenol using nonionic surfactant self-organized media. Anal. Methods 2013, 5, 4750–4756. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Ziganshina, E.; Romashkina, S.; Budnikov, H. Highly sensitive amperometric sensor for eugenol quantification based on CeO2 nanoparticles and surfactants. Electroanalysis 2017, 29, 1197–1204. [Google Scholar] [CrossRef]
- Maciel, J.V.; Silva, T.A.; Dias, D.; Fatibello-Filho, O. Electroanalytical determination of eugenol in clove oil by voltammetry of immobilized microdroplets. J. Solid State Electrochem. 2018, 22, 2277–2285. [Google Scholar] [CrossRef]
- Kowalcze, M.; Wyrwa, J.; Dziubaniuk, M.; Jakubowska, M. Voltammetric determination of anethole on La2O3/CPE and BDDE. J. Anal. Methods Chem. 2018, 2018, 2158407. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Antonova, T.S.; Mubarakova, L.R.; Budnikov, H.C. An amperometric sensor based on tin dioxide and cetylpyridinium bromide nanoparticles for the determination of vanillin. J. Anal. Chem. 2018, 73, 801–808. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, S.; Wang, L.; Yang, G. Electroanalysis of ascorbic acid using poly(bromocresol purple) film modified glassy carbon electrode. Measurement 2013, 46, 1089–1093. [Google Scholar] [CrossRef]
- Banu, R.; Swamy, B.E.K. Poly (Bromocresol purple) incorporated pencil graphite electrode for concurrent determination of serotonin and levodopa in presence of L-Tryptophan: A voltammetric study. Inorg. Chem. Commun. 2022, 141, 109495. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Guss, E.; Morozova, E.; Budnikov, H.; Davletshin, R.; Vorobev, V.; Osin, Y. Simultaneous voltammetric determination of gallic and ellagic acids in cognac and brandy using electrode modified with functionalized SWNT and poly(pyrocatechol violet). Food Anal. Methods 2019, 12, 2250–2261. [Google Scholar] [CrossRef]
- Taei, M.; Hasanpour, F.; Tavakkoli, N.; Bahrameian, M. Electrochemical characterization of poly(fuchsine acid) modified glassy carbon electrode and its application for simultaneous determination of ascorbic acid, epinephrine and uric acid. J. Mol. Liq. 2015, 211, 353–362. [Google Scholar] [CrossRef]
- Promsuwan, K.; Kaewjunlakan, C.; Saichanapan, J.; Soleh, A.; Saisahas, K.; Thipwimonmas, Y.; Kongkaew, S.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Poly(phenol red) hierarchical micro-structure interface enhanced electrode kinetics for adsorption and determination of hydroquinone. Electrochim. Acta 2021, 377, 138072. [Google Scholar] [CrossRef]
- Zhupanova, A.; Guss, E.; Ziyatdinova, G.; Budnikov, H. Simultaneous voltammetric determination of flavanones using an electrode based on functionalized single-walled carbon nanotubes and polyaluminon. Anal. Lett. 2020, 53, 2170–2189. [Google Scholar] [CrossRef]
- Guss, E.V.; Ziyatdinova, G.K.; Zhupanova, A.S.; Budnikov, H.C. Voltammetric determination of quercetin and rutin in their simultaneous presence on an electrode modified with polythymolphthalein. J. Anal. Chem. 2020, 75, 526–535. [Google Scholar] [CrossRef]
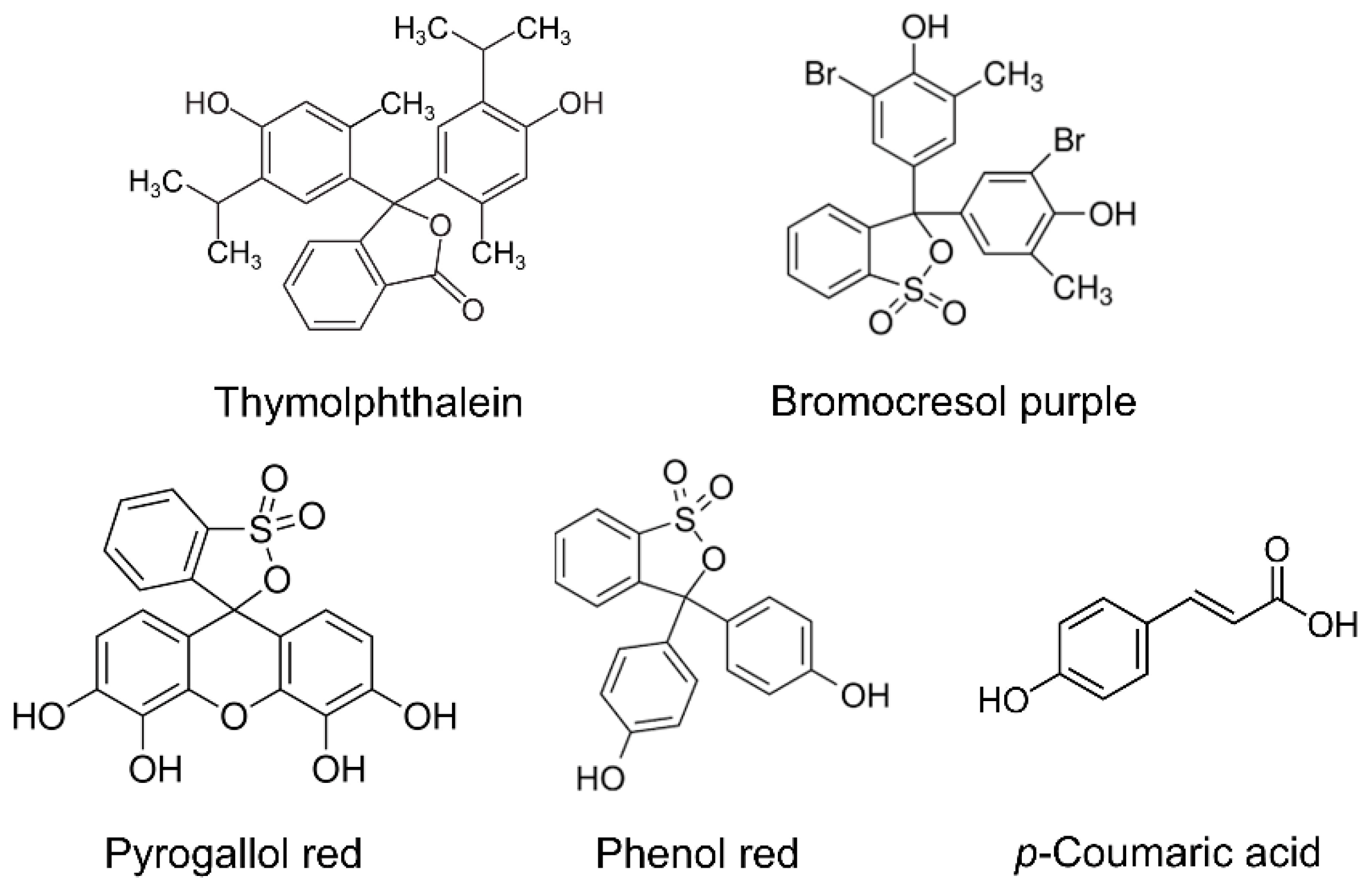
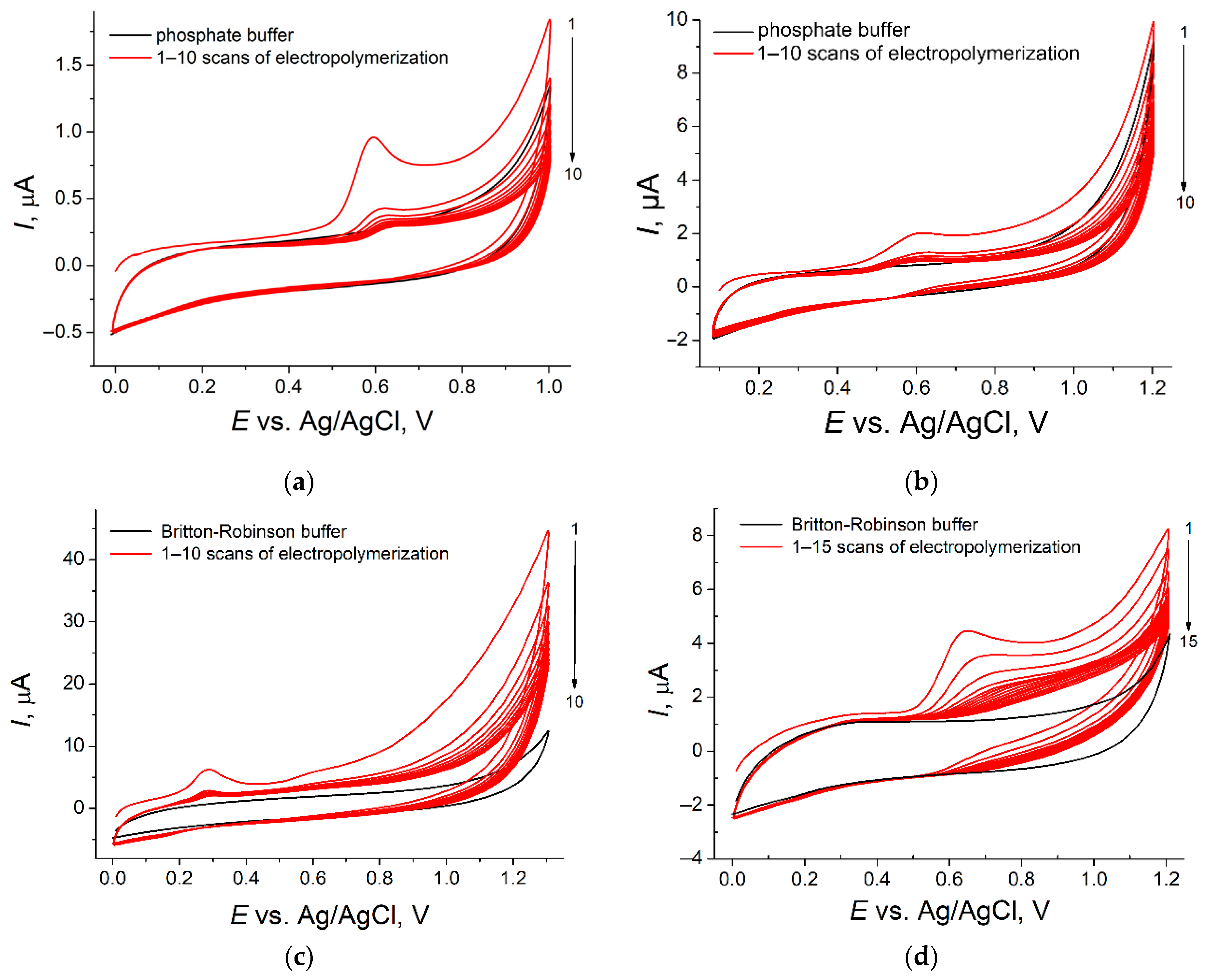
| Sensing Layer | c, M | Number of Scans | Potential Range, V | υ, mV s−1 | Supporting Electrolyte |
|---|---|---|---|---|---|
| Poly(thymolphthalein)/MWCNTs | 1.0 × 10−5 | 10 | 0.0–1.0 | 100 | 0.1 M phosphate buffer pH 7.0 |
| Poly(bromocresol purple)/SWCNTs-f | 2.5 × 10−5 | 10 | 0.0–1.2 | 100 | |
| Poly(pyrogallol red)/Carboxylated MWCNTs | 1.0 × 10−4 | 10 | 0.0–1.3 | 75 | Britton–Robinson buffer pH 7.0 |
| Poly(phenol red–co–p-coumaric acid)/MWCNTs | 1.0 × 10−4 | 15 | 0.0–1.2 | 50 |
| Sensor | Analyte | Method | Eox, V | Linear Dynamic Range, M | Detection Limit, M |
|---|---|---|---|---|---|
| Poly(thymolphthalein)/MWCNTs/GCE | Thymol | DPV 1 | 0.81 | 5.0 × 10−8–2.5 × 10−5 2.5 × 10−5–1.0 × 10−4 | 3.7 × 10−8 |
| Carvacrol | 0.83 | 1.0 × 10−7–1.0 × 10−5 1.0 × 10−5–1.0 × 10−4 | 6.3 × 10−8 | ||
| Poly(bromocresol purple)/SWCNTs-f/GCE | Vanillin | DPV | 0.86 | 1.0 × 10−7–5.0 × 10−6 5.0 × 10−6–2.5 × 10−5 | 6.4 × 10−8 |
| Poly(pyrogallol red)/Carboxylated MWCNTs/GCE | Eugenol | DPV | 0.57 | 7.5 × 10−7–1.0 × 10−4 | 7.3 × 10−7 |
| Poly(phenol red–co–p-coumaric acid)/MWCNTs/GCE | trans-Anethole | AdDPV 2 | 0.95 | 1.0 × 10−7–7.5 × 10−6 7.5 × 10−6–7.5 × 10−5 | 9.5 × 10−8 |
| Interference | Tolerance Limit, M | |||
|---|---|---|---|---|
| 1.0 × 10−6 M Thymol or Carvacrol | 1.0 × 10−6 M Vanillin | 5.0 × 10−6 M Eugenol | 1.0 × 10−6 M trans-Anethole | |
| K+, Mg2+, Ca2+, NO3−, Cl−, SO42− | 1.0 × 10−3 | 1.0 × 10−3 | 5.0 × 10−3 | 1.0 × 10−3 |
| Glucose, rhamnose, sucrose | 1.0 × 10−4 | 1.0 × 10−4 | 5.0 × 10−4 | 1.0 × 10−4 |
| Thymol | — | 0 | 2.5 × 10−5 | 2.5 × 10−7 |
| Cavacrol | — | 0 | 5.0 × 10−5 | 2.5 × 10−7 |
| Vanillin | 0 | — | 5.0 × 10−4 | 0 |
| trans-Anethole | 1.0 × 10−7 | 0 | 5.0 × 10−4 | — |
| Eugenol | 5.0 × 10−6 | 1.0 × 10−4 | — | 1.5 × 10−5 |
| α-Pinene | 1.0 × 10−4 | 5.0 × 10−5 | 5.0 × 10−4 | 2.5 × 10−7 |
| Limonene | 1.0 × 10−4 | 5.0 × 10−5 | 5.0 × 10−4 | 5.0 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalmykova, A.; Zhupanova, A.; Ziyatdinova, G. Electropolymerized Dyes as Sensing Layer for Natural Phenolic Antioxidants of Essential Oils. Eng. Proc. 2024, 82, 18. https://doi.org/10.3390/ecsa-11-20480
Kalmykova A, Zhupanova A, Ziyatdinova G. Electropolymerized Dyes as Sensing Layer for Natural Phenolic Antioxidants of Essential Oils. Engineering Proceedings. 2024; 82(1):18. https://doi.org/10.3390/ecsa-11-20480
Chicago/Turabian StyleKalmykova, Alena, Anastasiya Zhupanova, and Guzel Ziyatdinova. 2024. "Electropolymerized Dyes as Sensing Layer for Natural Phenolic Antioxidants of Essential Oils" Engineering Proceedings 82, no. 1: 18. https://doi.org/10.3390/ecsa-11-20480
APA StyleKalmykova, A., Zhupanova, A., & Ziyatdinova, G. (2024). Electropolymerized Dyes as Sensing Layer for Natural Phenolic Antioxidants of Essential Oils. Engineering Proceedings, 82(1), 18. https://doi.org/10.3390/ecsa-11-20480







