Eggshell Waste Valorization into CaO/CaCO3 Solid Base Catalysts †
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Materials
2.2. Characterization
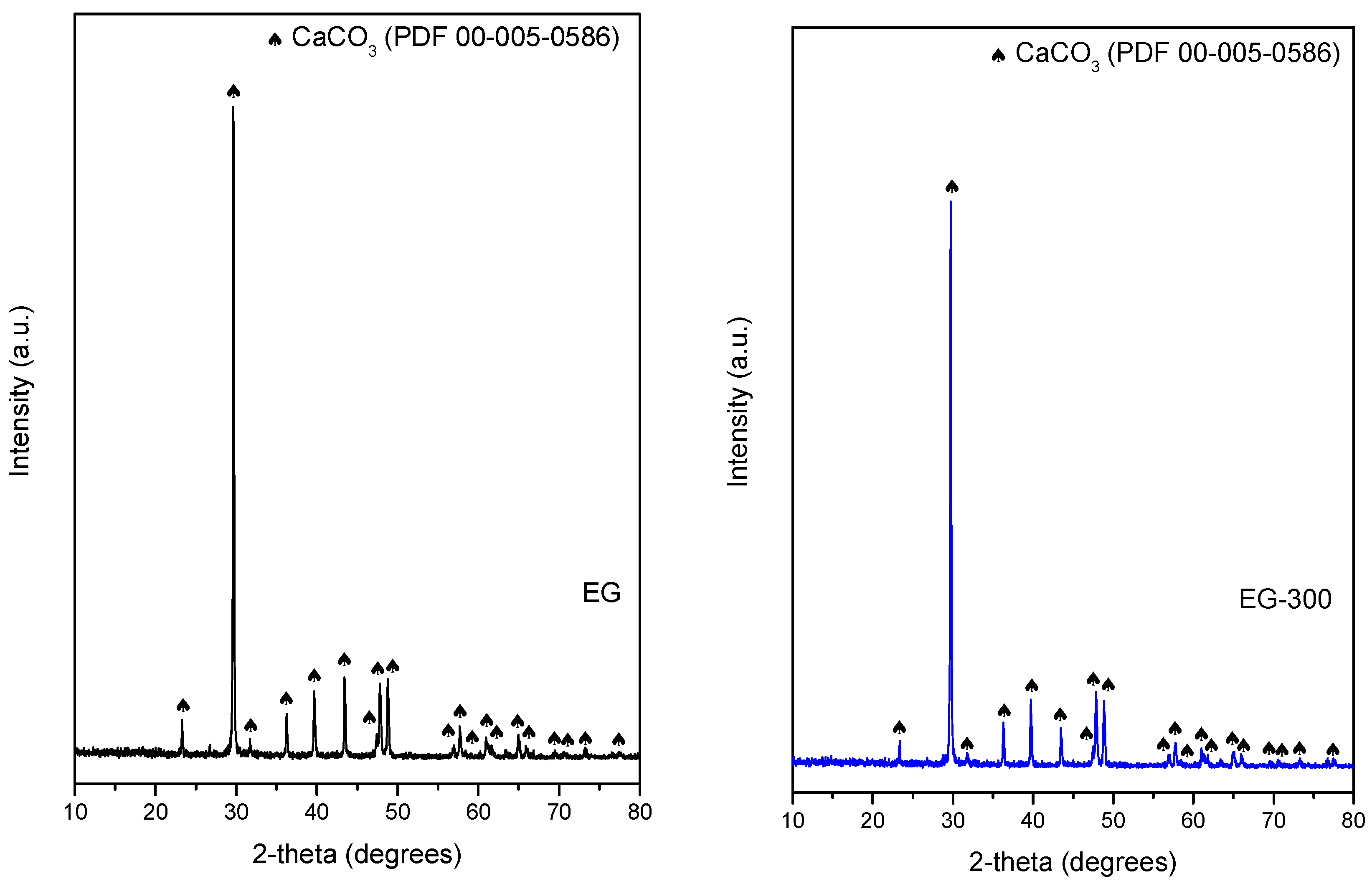
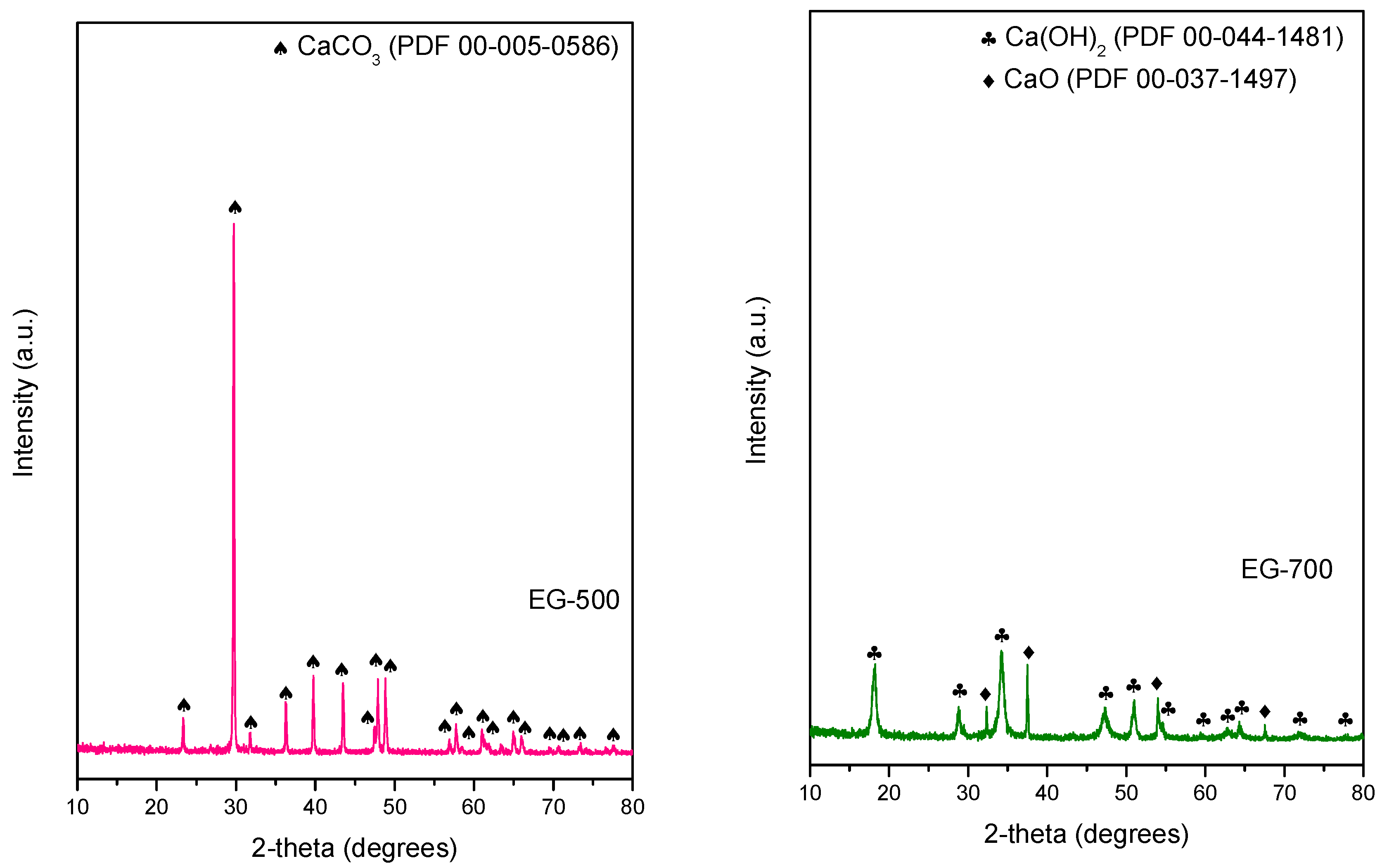
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Z.; Xu, C.; Li, B. Application of waste eggshell as low-cost solid catalyst for biodiesel production. Bioresour. Technol. 2009, 100, 2883–2885. [Google Scholar] [CrossRef] [PubMed]
- Vandeginste, V. Food waste eggshell valorization through development of new composites: A review. Sustain. Mater. Technol. 2021, 29, e00317. [Google Scholar] [CrossRef]
- Boechat, C.L.; Arauco, A.M.d.; Duda, R.M.; Sena, A.F.S.d.; Souza, M.E.L.d.; Brito, A.C.C. Solid Waste in Agricultural Soils: An Approach Based on Environmental Principles, Human Health, and Food Security. In Solid Waste Management in Rural Areas; InTech: Penang, Malaysia, 2017. [Google Scholar] [CrossRef]
- Laca, A.; Laca, A.; Díaz, M. Eggshell waste as catalyst: A review. J. Environ. Manag. 2017, 197, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Waheed, M.; Yousaf, M.; Shehzad, A.; Inam-Ur-Raheem, M.; Khan, M.K.I.; Khan, M.R.; Ahmad, N.; Abdullah; Aadil, R.M. Channelling eggshell waste to valuable and utilizable products: A comprehensive review. Trends Food Sci. Technol. 2020, 106, 78–90. [Google Scholar] [CrossRef]
- Tangboriboon, N.; Kunanuruksapong, R.; Sirivat, A.; Kunanuruksapong, R.; Sirivat, A. Preparation and properties of calcium oxide from eggshells via calcination. Mater. Sci. 2012, 30, 313–322. [Google Scholar] [CrossRef]
- Kirubakaran, M.; Selvan, V.A.M. Experimental investigation on the effects of micro eggshell and nano-eggshell catalysts on biodiesel optimization from waste chicken fat. Bioresour. Technol. Rep. 2021, 14, 100658. [Google Scholar] [CrossRef]
- Quina, M.J.; Soares, M.A.R.; Quinta-Ferreira, R. Applications of industrial eggshell as a valuable anthropogenic resource. Resour. Conserv. Recycl. 2017, 123, 176–186. [Google Scholar] [CrossRef]
- Cree, D.; Pliya, P. Effect of elevated temperature on eggshell, eggshell powder and eggshell powder mortars for masonry applications. J. Build. Eng. 2019, 26, 100852. [Google Scholar] [CrossRef]
- Correia, L.M.; Saboya, R.M.A.; Campelo, N.d.; Cecilia, J.A.; Rodríguez-Castellón, E.; Cavalcante, C.L.; Vieira, R.S. Characterization of calcium oxide catalysts from natural sources and their application in the transesterification of sunflower oil. Bioresour. Technol. 2014, 151, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Jeong, W.G.; Park, J.; Baek, K. Partially calcined CaCO3 for remediating multi-heavy metals-contaminated groundwater. Chem. Eng. J. 2023, 471, 144652. [Google Scholar] [CrossRef]
- Tan, Y.H.; Abdullah, M.O.; Nolasco-Hipolito, C.; Taufiq-Yap, Y.H. Waste ostrich- and chicken-eggshells as heterogeneous base catalyst for biodiesel production from used cooking oil: Catalyst characterization and biodiesel yield performance. Appl. Energy 2015, 160, 58–70. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaudo, M.G.; Collins, S.E.; Morales, M.R. Eggshell Waste Valorization into CaO/CaCO3 Solid Base Catalysts. Eng. Proc. 2024, 67, 36. https://doi.org/10.3390/engproc2024067036
Rinaudo MG, Collins SE, Morales MR. Eggshell Waste Valorization into CaO/CaCO3 Solid Base Catalysts. Engineering Proceedings. 2024; 67(1):36. https://doi.org/10.3390/engproc2024067036
Chicago/Turabian StyleRinaudo, Matías G., Sebastián E. Collins, and Maria R. Morales. 2024. "Eggshell Waste Valorization into CaO/CaCO3 Solid Base Catalysts" Engineering Proceedings 67, no. 1: 36. https://doi.org/10.3390/engproc2024067036
APA StyleRinaudo, M. G., Collins, S. E., & Morales, M. R. (2024). Eggshell Waste Valorization into CaO/CaCO3 Solid Base Catalysts. Engineering Proceedings, 67(1), 36. https://doi.org/10.3390/engproc2024067036






