Encapsulation of Aqueous Extract of Hancornia speciosa †
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Extraction and Determination of Phenolic Compounds
2.3. Physical Chemistry Characterization of Soy Lecithin
2.4. Encapsulation of Phenolic Extracts in Liposomes
2.4.1. Particle Size, Polydispersion Index, and Zeta Potential
2.4.2. Encapsulation Efficiency
2.4.3. Optical Microscopy
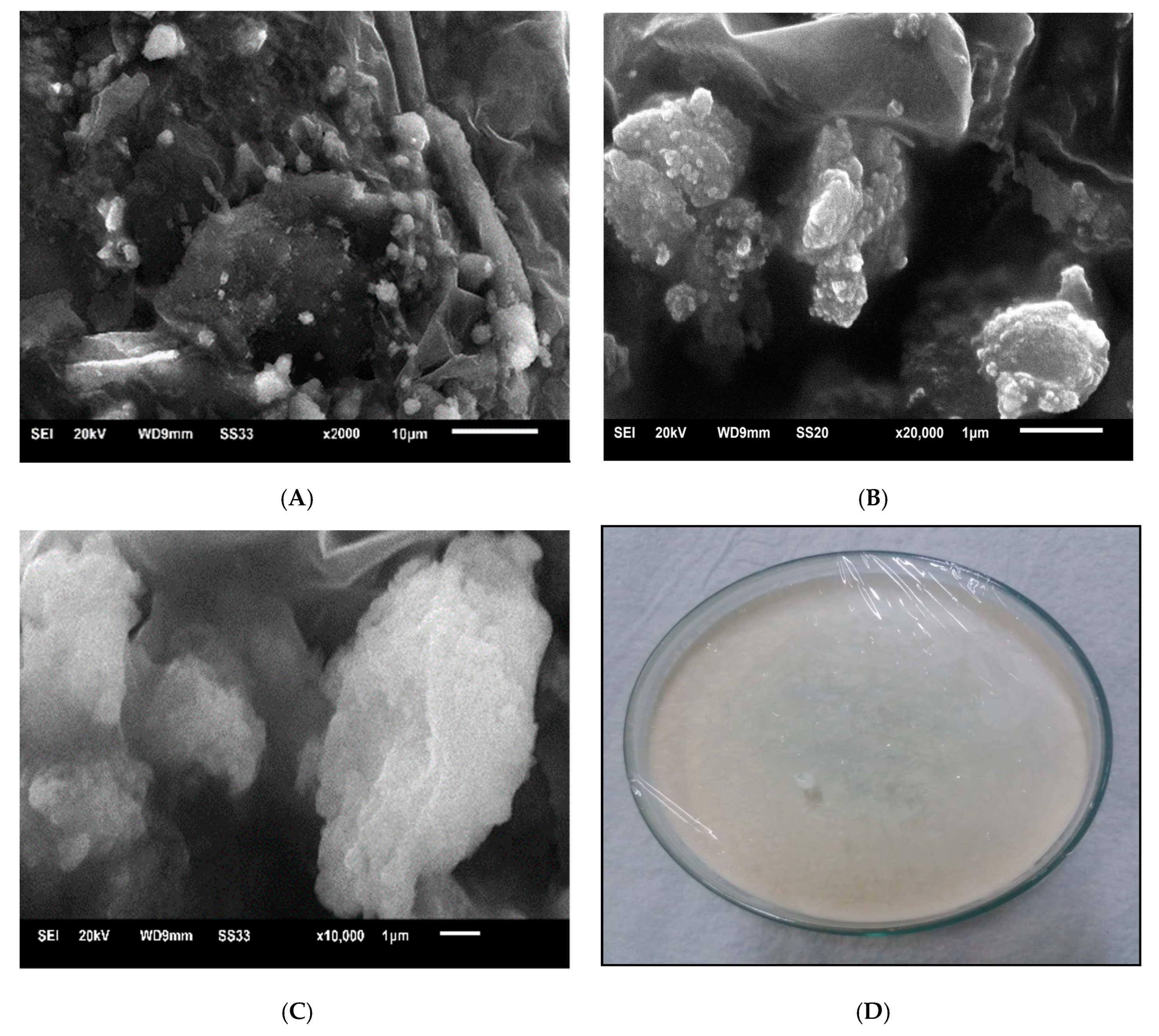
2.4.4. Scanning Electron Microscopy
2.5. Statistical Analysis
3. Results and Discussion
3.1. Quality Analysis of Soy Lecithin
| Quality Analysis | Outcome of the Study | Legislation Standards * |
|---|---|---|
| Moisture (%) | 0.35 | 0.40 |
| Acid value (mg/kg) | 0.06 | 29.00 |
| Peroxide index (meq/kg) | 0.20 | 5.00 |
| Insoluble in acetone (%) | 96.38 | 62.00 |
3.2. Physico-Chemical Characterization of Liposomes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Oliveira Filho, J.G.; de Sousa, T.L.; Bertolo, M.R.V.; Bogusz Junior, S.; Mattoso, L.H.C.; Pimentel, T.C.; Egea, M.B. Embalagens Para Alimentos de Última Geração: Filmes Bioativos Comestíveis Com Alginato, Polpa de Mangaba (Hancornia speciosa) e Saccharomyces boulardii. Food Biosci. 2023, 54, 102799. [Google Scholar] [CrossRef]
- Machado, A.; Assis, L.M.; Costa, J.A.; Badiale-Furlong, E.; Motta, A.; Micheletto, Y.M.S.; Souza-Soares, L. Application of Sonication and Mixing for Nanoencapsulation of the Cyanobacterium Spirulina Platensis in Liposomes. Int. Food Res. J. 2015, 22, 96–101. [Google Scholar]
- Machado, A.R.; Assis, L.M.; Machado, M.R.G.; Souza-Soares, L.A. Biological Evaluation of Casein and Spirulina in Rats and Encapsulation of These Protein Sources in Liposomes. Int. Food Res. J. 2015, 22, 344–350. [Google Scholar]
- Machado, A.R.; Pinheiro, A.C.; Vicente, A.A.; Souza-Soares, L.A.; Cerqueira, M.A. Liposomes Loaded with Phenolic Extracts of Spirulina LEB-18: Physicochemical Characterization and Behavior under Simulated Gastrointestinal Conditions. Food Res. Int. 2019, 120, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.R.; Machado, M.I.R.; de Souza-Soares, L.A. Avaliação do tamanho de particulas lipossomicas em diferentes ensaios. Res. Soc. Dev. 2023, 12, e24512135104. [Google Scholar] [CrossRef]
- Santos, L.S.d.; Medeiros, J.S.; Toledo, A.M.N.; Viana, L.F.; Machado, M.I.R.; Machado, A.R. Encapsulation process of lyophilized and non-lyophilized aqueous extract of mangaba in lipid vesicles. Braz. J. Dev. 2020, 6, 2180–2188. [Google Scholar] [CrossRef]
- Toniazzo, T.; Berbel, I.F.; Cho, S.; Fávaro-Trindade, C.S.; Moraes, I.C.F.; Pinho, S.C. β-Carotene-Loaded Liposome Dispersions Stabilized with Xanthan and Guar Gums: Physico-Chemical Stability and Feasibility of Application in Yogurt. LWT—Food Sci. Technol. 2014, 59, 1265–1273. [Google Scholar] [CrossRef]
- Souza, M. Potencial Antifúngico, Antioxidante e Inibidor Da Produção de Aflatoxina Por Extratos Fenólicos de Chlorella Sp. E Spirulina Platensis. 2012. Available online: https://bdtd.ibict.br/vufind/Record/FURG_19aeb2ea356735fd7c31eda19c011cae (accessed on 13 July 2018).
- American Oil Chemists Society. Official Methods and Recommended Practices, 4ed.; American Oil Chemists Society: Champaing, IL, USA, 1993; Volume 3. [Google Scholar]
- Mertins, O.; Sebben, M.; Henrique Schneider, P.; Pohlmann, A.R.; Silveira, N.P. da Caracterização da pureza de fosfatidilcolina da soja através de RMN de 1H e de 31P. Quím. Nova 2008, 31, 1856–1859. [Google Scholar] [CrossRef]
- Malvern. Dynamic Light Scattering: An Introduction in 30 Minutes. Version 4, Technical Note. 2014. Available online: http://www.malvern.com/br/support/resource-center/technical-notes/tn101104dynamiclightscatteringintroduction.aspx (accessed on 6 November 2018).
- de Assis, L.M.; Machado, A.R.; da Motta, A.D.S.; Costa, J.A.V.; de Souza-Soares, L.A. Development and Characterization of Nanovesicles Containing Phenolic Compounds of Microalgae Spirulina Strain LEB-18 and Chlorella Pyrenoidosa. Adv. Mater. Phys. Chem. 2014, 4, 42009. [Google Scholar] [CrossRef]
- Castejon, L.V. Parâmetros de Qualidade na Clarificação da Lecitina de Soja; Universidade Federal de Uberlândia: Uberlândia, Brazil, 2015. [Google Scholar]
- Zulian, S.L. Adição de Ácido Graxo de Soja Como Agente Estabilizante da Viscosidade de Lecitina de Soja. 2016. Available online: https://repositorio.ufsc.br/xmlui/handle/123456789/176058 (accessed on 12 February 2021).
- Sikorski, Z.Z.E.; Kolakowska, A. Chemical and Functional Properties of Food Lipids; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-4200-3199-7. [Google Scholar]
- Fernández Avila, C. Stability Assessment of Emulsions Treated by Ultra-High Pressure Homogenization and Their Incorporation in a Uht Milk-Based Product for Delivery of Conjugated Linoleic Acid. 2016. Available online: https://ddd.uab.cat/pub/tesis/2016/hdl_10803_399169/cfa1de1.pdf (accessed on 12 February 2021).
- Bordinassi, P.D.; Johann, G.; Coró, F.A.G.; Pedrão, M.R. Adição de Peróxido de Hidrogênio em lecitina de soja e análise de possíveis alterações de seus padrões Físico-Químicos. Res. Soc. Dev. 2022, 11, e50611629316. [Google Scholar] [CrossRef]
- Mezouari, S.; Eichner, K. Evaluation of the Stability of Blends of Sunflower and Rice Bran Oil. Eur. J. Lipid Sci. Technol. 2007, 109, 531–535. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Ropers, M.-H.; Genot, C. Lipid Oxidation in Oil-in-Water Emulsions: Involvement of the Interfacial Layer. Compr. Rev. Food Sci. Food Saf. 2014, 13, 945–977. [Google Scholar] [CrossRef]
- Esposto, B.S.; Jauregi, P.; Tapia-Blácido, D.R.; Martelli-Tosi, M. Liposomes vs. Chitosomes: Encapsulating Food Bioactives. Trends Food Sci. Technol. 2021, 108, 40–48. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, B.; Jaafari, M.R.; Golabpour, A.; Momtazi-Borojeni, A.A.; Karimi, M.; Eslami, S. Application of Ensemble Machine Learning Approach to Assess the Factors Affecting Size and Polydispersity Index of Liposomal Nanoparticles. Sci. Rep. 2023, 13, 18012. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.; Pal, A.K.; Jambhrunkar, S.; Patel, P.; Thakur, S.S.; Reátegui, E.; Parekh, H.S.; Saá, P.; Stassinopoulos, A.; Broom, M.F. High-Resolution Single Particle Zeta Potential Characterisation of Biological Nanoparticles Using Tunable Resistive Pulse Sensing. Sci. Rep. 2017, 7, 17479. [Google Scholar] [CrossRef] [PubMed]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef] [PubMed]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta Potential of Extracellular Vesicles: Toward Understanding the Attributes That Determine Colloidal Stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef] [PubMed]
| Physical Characterization | Concentration (mg/mL) | ||
|---|---|---|---|
| 1.0 | 1.5 | 2.0 | |
| Particle diameter (nm) | 197.43 ± 0.81 c | 318.2 ± 1.55 a | 238.33 ± 0.89 b |
| Polydispersion | 0.28 ± 0.03 c | 0.49 ± 0.01 a | 0.47 ± 0.02 a |
| Zeta Potential | −37.00 ± 0.17 a | −33.7 ± 1.48 b | −35.7 ± 0.87 b |
| Encapsulation Efficiency (%) | 80.14 ± 0.07 c | 86.18 ± 0.78 b | 88.09 ± 0.45 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, L.S.; Medeiros, J.S.; de Toledo, A.M.N.; Viana, L.F.; Machado, M.I.R.; Machado, A.R. Encapsulation of Aqueous Extract of Hancornia speciosa . Eng. Proc. 2024, 67, 22. https://doi.org/10.3390/engproc2024067022
dos Santos LS, Medeiros JS, de Toledo AMN, Viana LF, Machado MIR, Machado AR. Encapsulation of Aqueous Extract of Hancornia speciosa . Engineering Proceedings. 2024; 67(1):22. https://doi.org/10.3390/engproc2024067022
Chicago/Turabian Styledos Santos, Lorrane Soares, Jéssica Silva Medeiros, Antonio Matias Navarrete de Toledo, Letícia Fleury Viana, Maria Inês Rodrigues Machado, and Adriana Rodrigues Machado. 2024. "Encapsulation of Aqueous Extract of Hancornia speciosa " Engineering Proceedings 67, no. 1: 22. https://doi.org/10.3390/engproc2024067022
APA Styledos Santos, L. S., Medeiros, J. S., de Toledo, A. M. N., Viana, L. F., Machado, M. I. R., & Machado, A. R. (2024). Encapsulation of Aqueous Extract of Hancornia speciosa . Engineering Proceedings, 67(1), 22. https://doi.org/10.3390/engproc2024067022





