Abstract
This study is dedicated to an investigation and comparison of the kinetics of the foam stability of protein foaming agents based on the hydrolysates of solid keratin. The work utilized ready-made hydrolysates based on sodium hydroxide and a mixture of sodium hydroxide with calcium hydroxide for the synthesis of foaming agents. The synthesis was carried out according to the author’s methodology. Among the indicators studied were foam multiplication, foam specific weight, foam stability over time, and average foam stability reduction rate. Experiments were conducted with various concentrations and ratios of components at constant temperature, pressure, and pH values, as well as mixing speed, mixing time, and observation time. Itis hypothesized that protein foaming agents based on hydrolysates of solid keratin using a mixture of hydroxides will not be able to achieve optimal values in the kinetics of foam stability. In contrast, protein foaming agents based on hydrolysates of solid keratin using sodium hydroxide individually have high potential foaming properties and, consequently, good foam stability kinetics indicators. The results of this study may be useful in the development of new synthesis methods for protein foaming agents with optimal foaming properties or for improving those that already exist. The research itself and the products obtained during it—protein foaming agents—are mainly aimed at expanding the industrial sphere of human activity. This may also have practical applications in other areas, such as the food industry, cosmetology, medicine, and others.
1. Introduction
Nowadays, human activity is accompanied by the formation of a huge amount of secondary biological waste, which pollutes the environment; however, this waste remains a very important resource in terms of other valuable products. The problem of the complex processing of raw materials, with its involvement in industrial and economic turnover in terms of the production of waste as a by-product, has become very relevant and significant when considering the scale of modern industry [1,2,3].
In recent years, the volume of work and research aimed at studying multi-component systems and raw materials of animal origin has increased dramatically, and a large number of ways to extract valuable chemical products from these materials have appeared. Keratin, a protein with increased strength, is mainly obtained. Protein blowing agents based on hydrolysates of keratin-containing raw materials obtained using alkaline hydrolysis have properties indispensable for the construction industry. For example, protein blowing agents can be used to produce foamed concrete with the highest quality characteristics [4].
The synthesis of blowing agents based on keratin-containing raw materials is an effective way to recycle secondary raw materials to obtain products useful to society, such as building materials. We have carried out a unique synthesis of these compounds with a subsequent evaluation of their properties. The development of the production of blowing agents is an urgent task and has great prospects for the expansion of production [5].
2. Materials and Methods
The main component of the protein blowing agent is hydrolysate, which is obtained via the alkaline hydrolysis of keratin-containing raw materials. The foaming properties of a protein blowing agent depend on many factors, including the parameters of the hydrolysis process of keratin-containing raw materials, namely, the origin of keratin-containing raw materials, the nature of the alkaline hydrolyzing reagent and its concentration in the hydrolyzing solution, and the hydrolysis conditions of keratin-containing raw materials. The component composition of the protein blowing agent is also important [6].
2.1. Raw Materials and Reagents for Preparation
For the preparation of protein blowing agents, we used hydrolysates of keratin-containing raw materials obtained under laboratory conditions via the alkaline hydrolysis of keratin-containing raw materials: horny hoof raw material (HHRM) using sodium hydroxide as a hydrolyzing agent, as well as a mixture of sodium hydroxide and calcium hydroxide. The component composition included protein hydrolysate, hydrochloric acid, hydrogen peroxide, magnesium chloride, water, iron (II) sulfate crystallohydrate, isobutyl alcohol and others.
2.2. Products Obtained
The methodology used for the preparation of protein blowing agents under laboratory conditions is based on the sequence of operations used in the production conditions, and it was adapted to the conditions of the laboratory experiment.
In our work, we used already ready hydrolysates on the basis of keratin-containing raw materials (HHRM), from which we obtained 11 protein blowing agents; in 6 of them, sodium hydroxide (NaOH) was the alkaline agent during hydrolysis, and in the remaining 5, a mixture of sodium hydroxide and calcium hydroxide (Ca(OH)2). The characterization of these hydrolysates is presented in Table 1.

Table 1.
Characterization of the obtained blowing agents.
After following the production methodology for BA assembly, it is recommended to infuse each blowing agent for 24 h and, after that, to obtain the foam and to determine the foam multiplicity, specific gravity, and foam stability.
3. Results and Discussion
3.1. Analysis of Foam Stability Kinetics
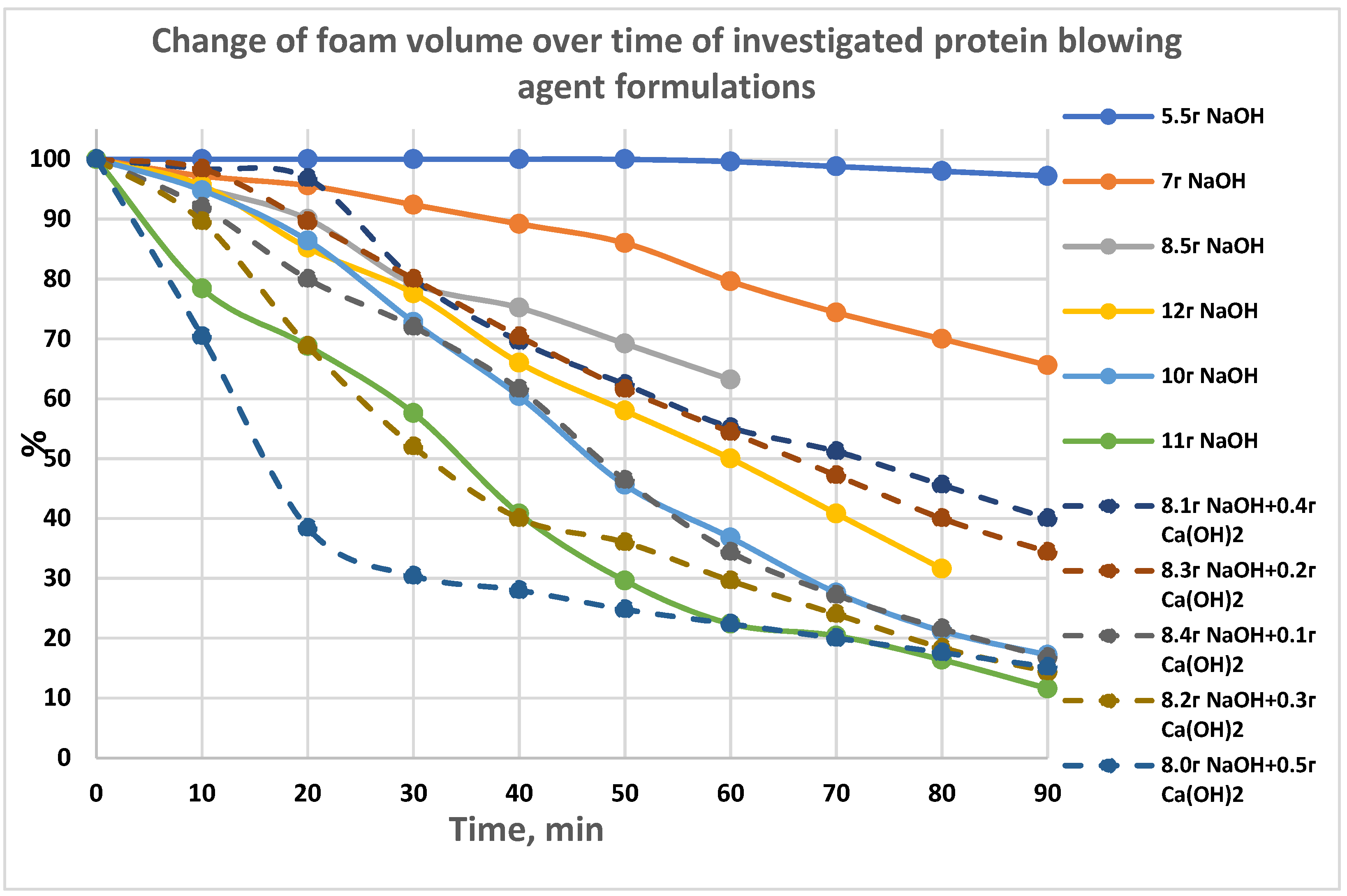
The foam stability of blowing agent solution (BA) foam is of great importance in the process of making foam concrete, which is evaluated by examining the change in foam volume over time, and it was determined by using the ratio of the foam volume at the current time to the initial time. The foam should be stable for 0.5–1 h. We recorded the foam volume every 10 min for 1.5 h in most cases. The initial foam volume was taken as 100%, and for each measurement, the foam volume was calculated as a percentage of the initial volume. Figure 1 shows the time dependences of foam volume change (kinetics) for the investigated blowing agent solutions depending on the content of hydrolyzing agent or a mixture of hydrolyzing agents.
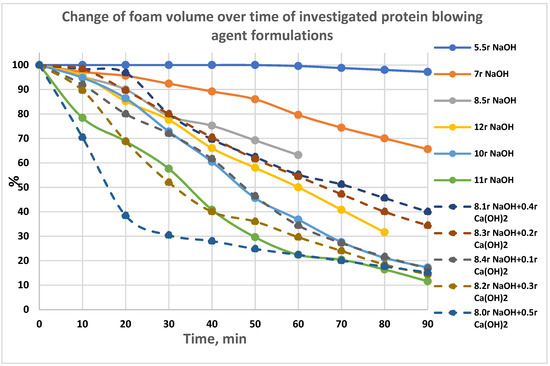
Figure 1.
The change of foam volume over time of investigated protein blowing agent formulations.
3.2. Determination of the Average Rate of Foam Stability Decrease
It was decided to determine the average rates of decrease in foam stability, which are given in Table 2, as arithmetic mean values for the whole time of the experiment.

Table 2.
Average rates of foam stability reduction for the investigated blowing agent solutions.
The average rate of decreasing foam stability is defined as the ratio of the difference between the initial and final foam volumes, in %, and the duration of foam stability determination. Based on the results shown in Table 2, we can conclude that the slowest to lose its volume of foam solutions BA No. 1 and 2. The others, in turn, lose approximately 1% of their foam volume per minute.
4. Conclusions
Thus, under the experimental conditions, it was found that reducing the ratio of the mass of hydrolyzing agent(s) to the mass of HHRM used in the hydrolysis of HHRM to a certain value leads to an increase in the stability of foam and the achievement of optimal values. Under the conditions of the experiment, it was found that the optimal of the 11 compositions studied can be called the composition of protein foaming agents prepared on the basis of hydrolyzed HHRM using sodium hydroxide in the ratio mNaOH:mHHRM.:mw, equal to 5.5:43:130.
Other alkaline reagents can also be used in the alkaline hydrolysis of keratin-containing raw materials. The data obtained in the present work suggest that when using other alkaline reagents, it is also necessary to reduce the content of alkaline reagents in the hydrolysis of keratin-containing raw materials to a certain value to achieve the required indicators of the foaming ability of protein blowing agents prepared on the basis of hydrolysates.
Author Contributions
Conceptualization, E.N.R. and V.M.Z.; methodology, E.N.R., K.A.B. and V.M.Z.; software, K.A.B. and E.S.B.; validation, E.N.R. and K.A.B.; formal analysis, K.A.B. and E.N.R.; investigation, K.A.B. and E.N.R.; resources, E.N.R. and V.M.Z.; data curation, E.N.R. and K.A.B.; writing—original draft preparation, K.A.B., E.S.B. and E.N.R.; writing—review and editing, K.A.B., E.S.B. and E.N.R.; visualization, K.A.B.; supervision, E.N.R. and V.M.Z.; project administration, E.N.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zuev, V.V. Physics and Chemistry of Polymers; ITMO: Saint Petersburg, Russia, 2010; pp. 45–48. [Google Scholar]
- Khristov, K.; Exerowa, D.; Kruglyakov, P.M. Multilayer foam films and foams from surfactant solutions with high solubilizing ability. Chemistry 1993, 78, 221–227. [Google Scholar] [CrossRef]
- Katanov, N.F. Obtaining Keratin Protein Products from Feather and Down Waste; Khakassky State University: Abakan, Russia, 2019; p. 4. [Google Scholar]
- Vilkova, N.G. Properties of Foams and Methods of Their Research: A Monograph; PSUAS: Penza, Russia, 2013; p. 120. [Google Scholar]
- Schitz, L.A. Surface-Active Substances; Bolshaya Rossiyskaya Encyclopedia: Moscow, Russia, 2014; p. 487. [Google Scholar]
- Veslava, M. Degradation of keratin containing wastes by bacteria with keratinolytic activity. In Proceedings of the 7th International Scientific and Practical Conference, Rēzekne, Latvia, 25–27 June 2009; Volume 1, pp. 284–289. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).