Initial Assessment of Separation Train Design and Utilities Consumption for Cyclopentyl Methyl Ether Production †
Abstract
:1. Introduction
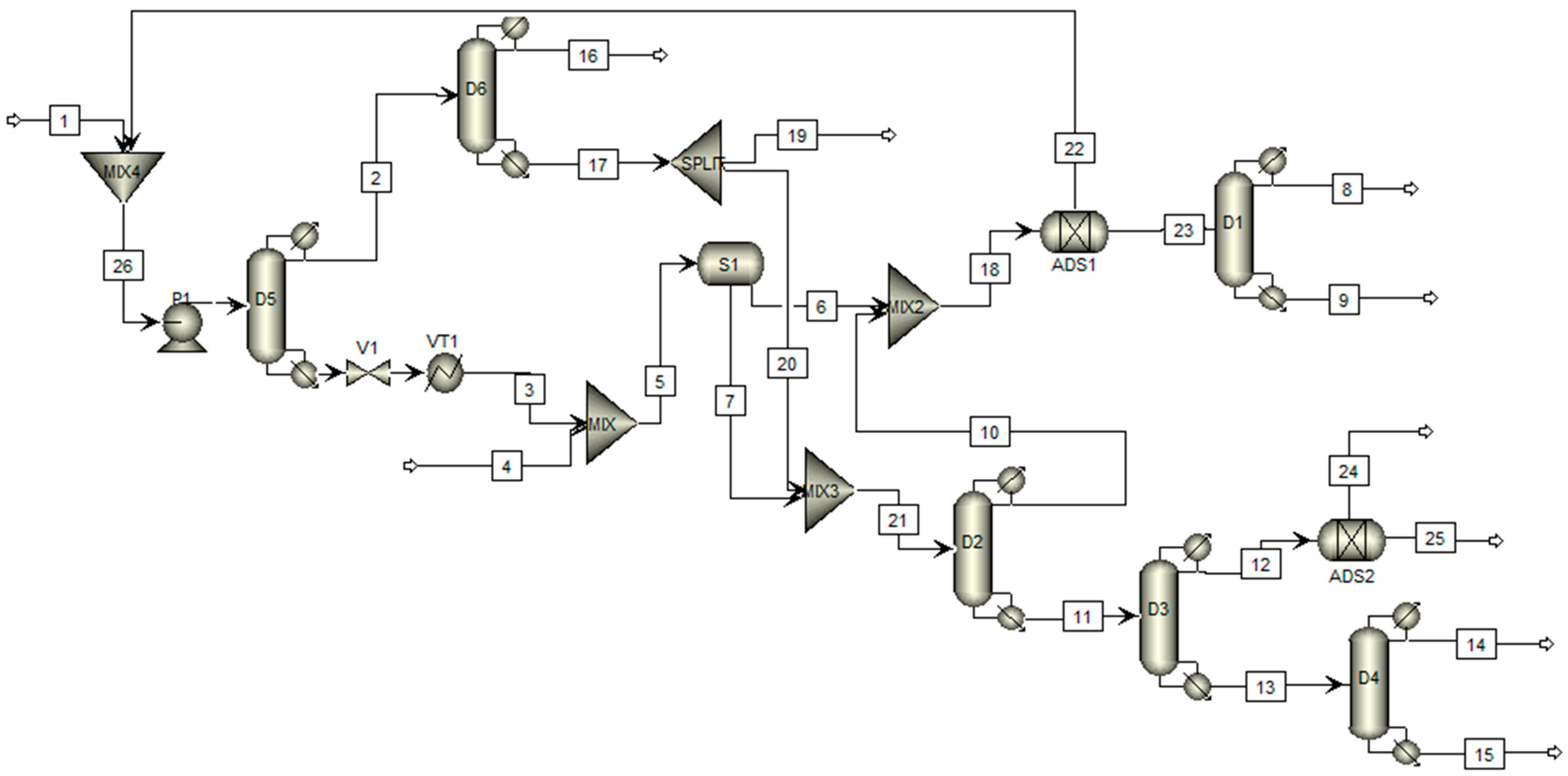
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azzena, U.; Carraro, M.; Pisano, L.; Monticelli, S.; Bartolotta, R.; Pace, V. Cyclopentyl Methyl Ether: An Elective Ecofriendly Ethereal Solvent in Classical and Modern Organic Chemistry. ChemSusChem 2019, 12, 40–70. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Yamagiwa, N.; Torisawa, Y. Cyclopentyl Methyl Ether as a New and Alternative Process Solvent. Org. Process Res. Dev. 2007, 11, 251–258. [Google Scholar] [CrossRef]
- Soták, T.; Magyarová, Z.; Shamzhy, M.; Kubů, M.; Gołąbek, K.; Čejka, J.; Hronec, M. Gas-phase etherification of cyclopentanol with methanol to cyclopentyl methyl ether catalyzed by zeolites. Appl. Catal. Gen. 2021, 618, 118122. [Google Scholar] [CrossRef]
- Miki, H. Synthesis of Cyclopentyl Methyl Ether by Gas Phase Catalytic Reaction Using Strong Acid Ion Exchange Resin as a Catalyst—Study of Catalyst Deactivation Mechanism. J. Jpn. Pet. Inst. 2019, 62, 173–180. [Google Scholar] [CrossRef]
- Pšidová, L. Príprava Éterov v Prítomnosti Heterogénnych Katalyzátorov. (Synthesis of Ethers in the Presence of Heterogeneous Catalysts, in Slovak). Master’s Thesis, Slovak University of Technology in Bratislava, Bratislava, Slovakia, 2020. Available online: https://opac.crzp.sk/?fn=detailBiblioFormChildI47RS&sid=F50A3640C856E51084E2BE61DB62&seo=CRZP-detail-kniha (accessed on 12 July 2023).
- Hönicke, D.; Födisch, R.; Claus, P.; Olson, M. Cyclopentadiene and Cyclopentene. In Ullmann’s Encyclopedia of Industrial Chemistry; KGaA, Ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Azizi, Z.; Rezaeimanesh, M.; Tohidian, T.; Rahimpour, M.R. Dimethyl ether: A review of technologies and production challenges. Chem. Eng. Process. Process Intensif. 2014, 82, 150–172. [Google Scholar] [CrossRef]
- Kim, S.A.; Yim, J.H.; Byun, H.-S.; Lim, J.S. Isothermal vapor-liquid equilibria for the binary system of dimethyl ether (CH3OCH3)+ methanol (CH3OH). Korean J. Chem. Eng. 2011, 28, 2324–2328. [Google Scholar] [CrossRef]
- Álvarez, V.H.; Mattedi, S.; Iglesias, M.; Gonzalez-Olmos, R.; Resa, J.M. Phase equilibria of binary mixtures containing methyl acetate, water, methanol or ethanol at 101.3 kPa. Phys. Chem. Liq. 2011, 49, 52–71. [Google Scholar] [CrossRef]
- Zeng, J.; Fu, C.; Hu, W.L. Study on the VLE for MethylFormate-Methanol System. Tianranqi Huagong 2000, 25, 54–56. [Google Scholar]
- Jones, D.E.G.; Weeks, I.A.; Anand, S.C.; Wetmore, R.W.; Benson, G.C. Thermodynamic properties of some cycloalkane-cycloalkanol systems at 25.deg. J. Chem. Eng. Data 1972, 17, 501–506. [Google Scholar] [CrossRef]
| Component | Abbreviation/Formula | Reaction Selectivity [%] | Molar Mass [g/mol] | Normal Boiling Point [°C] | Reactor Effluent [mol/h] |
|---|---|---|---|---|---|
| Cyclopentanol | CYPol | - | 86.13 | 139.85 | 540.94 |
| Methanol | MeOH | - | 32.04 | 64.6 | 23,634.99 |
| Cyclopentylmethylether | CPME | 65.8 | 100.16 | 106 | 1050.94 |
| Cyclopentene | CYPen | 21.7 | 68.12 | 43.85 | 346.59 |
| Water | H2O | - | 18.02 | 100 | 3532.16 |
| Dicyclopentylether | DCPE | 11.4 | 154.25 | 207.83 | 91.04 |
| 1-cyclopentylcyclopentene | CPCPen | 1 | 136.24 | 191.2 | 7.99 |
| Dimethyether | DME | 14.1 1 | 46.07 | −24.85 | 2026.03 |
| Soot | Approximated as C25H40 [5] | 0.1 | 340.60 | - | 0.32 |
| Stream/Component | Molar Flow [kmol/h] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 8 | 9 | 16 | 19 | 14 | 15 | 24 | 25 | |
| CYPol | 0.541 | 0 | 0 | 0 | 0 | 0 | 0.540 | 0.001 | 0 | 0 |
| MeOH | 23.635 | 0 | 23.598 | 0.024 | 0 | 0.002 | 0 | 0 | 0 | 0.011 |
| CPME | 1.056 | 0 | 0 | 0 | 0 | 0 | 0.002 | 0 | 0.003 | 1.051 |
| CYPen | 0.347 | 0 | 0 | 0 | 0.0004 | 0.3461 | 0 | 0 | 0 | 0 |
| Water | 3.532 | 24.764 | 0.028 | 28.071 | 0 | 0 | 0 | 0 | 0.196 | 0 |
| CPCPen | 0.008 | 0 | 0 | 0 | 0 | 0 | 0 | 0.008 | 0 | 0 |
| DME | 2.023 | 0 | 0 | 0 | 2.021 | 0.002 | 0 | 0 | 0 | 0 |
| DCPE | 0.091 | 0 | 0 | 0 | 0 | 0 | 0 | 0.091 | 0 | 0 |
| Sum | 31.233 | 24.764 | 23.626 | 28.095 | 2.022 | 0.350 | 0.542 | 0.100 | 0.199 | 1.062 |
| Column/Parameter | D5 | D6 | D1 | D2 | D3 | D4 |
|---|---|---|---|---|---|---|
| Reflux ratio | 3.79 | 0.49 | 0.67 | 3.01 | 0.83 | 0.73 |
| Number of theoretical plates | 33 | 27 | 14 | 81 | 35 | 20 |
| Condenser duty [kW] | 73.2 | 15.8 | 72.6 | 419.8 | 21.7 | 10.7 |
| Reboiler duty [kW] | 182.5 | 17.0 | 78.2 | 475.2 | 22.8 | 11.1 |
| Column head temperature [°C] | 38.8 | 30.7 | 49.6 | 65.4 | 77.4 | 138.8 |
| Column bottom temperature [°C] | 127.2 | 115.0 | 92.1 | 98.6 | 143.6 | 196.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Variny, M.; Hlavatý, L.; Soták, T.; Magyarová, Z. Initial Assessment of Separation Train Design and Utilities Consumption for Cyclopentyl Methyl Ether Production. Eng. Proc. 2023, 56, 57. https://doi.org/10.3390/ASEC2023-15307
Variny M, Hlavatý L, Soták T, Magyarová Z. Initial Assessment of Separation Train Design and Utilities Consumption for Cyclopentyl Methyl Ether Production. Engineering Proceedings. 2023; 56(1):57. https://doi.org/10.3390/ASEC2023-15307
Chicago/Turabian StyleVariny, Miroslav, Lukas Hlavatý, Tomáš Soták, and Zuzana Magyarová. 2023. "Initial Assessment of Separation Train Design and Utilities Consumption for Cyclopentyl Methyl Ether Production" Engineering Proceedings 56, no. 1: 57. https://doi.org/10.3390/ASEC2023-15307
APA StyleVariny, M., Hlavatý, L., Soták, T., & Magyarová, Z. (2023). Initial Assessment of Separation Train Design and Utilities Consumption for Cyclopentyl Methyl Ether Production. Engineering Proceedings, 56(1), 57. https://doi.org/10.3390/ASEC2023-15307







