Anti-Inflammatory Drug Repurposing for Intranasal Delivery: Ketoprofen Nanoemulgel Development for the Treatment of Glioma †
Abstract
:1. Introduction
2. Materials and Methods
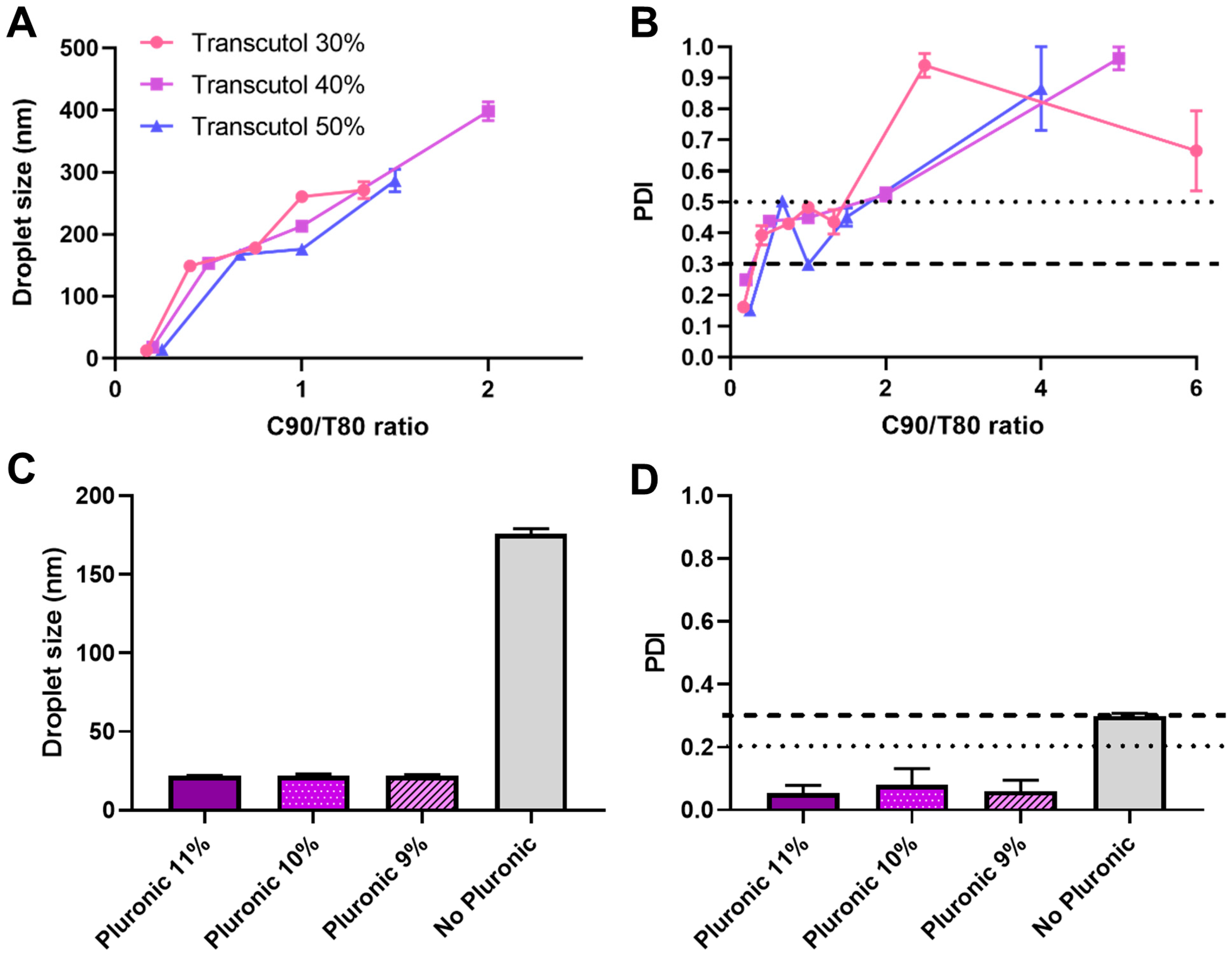
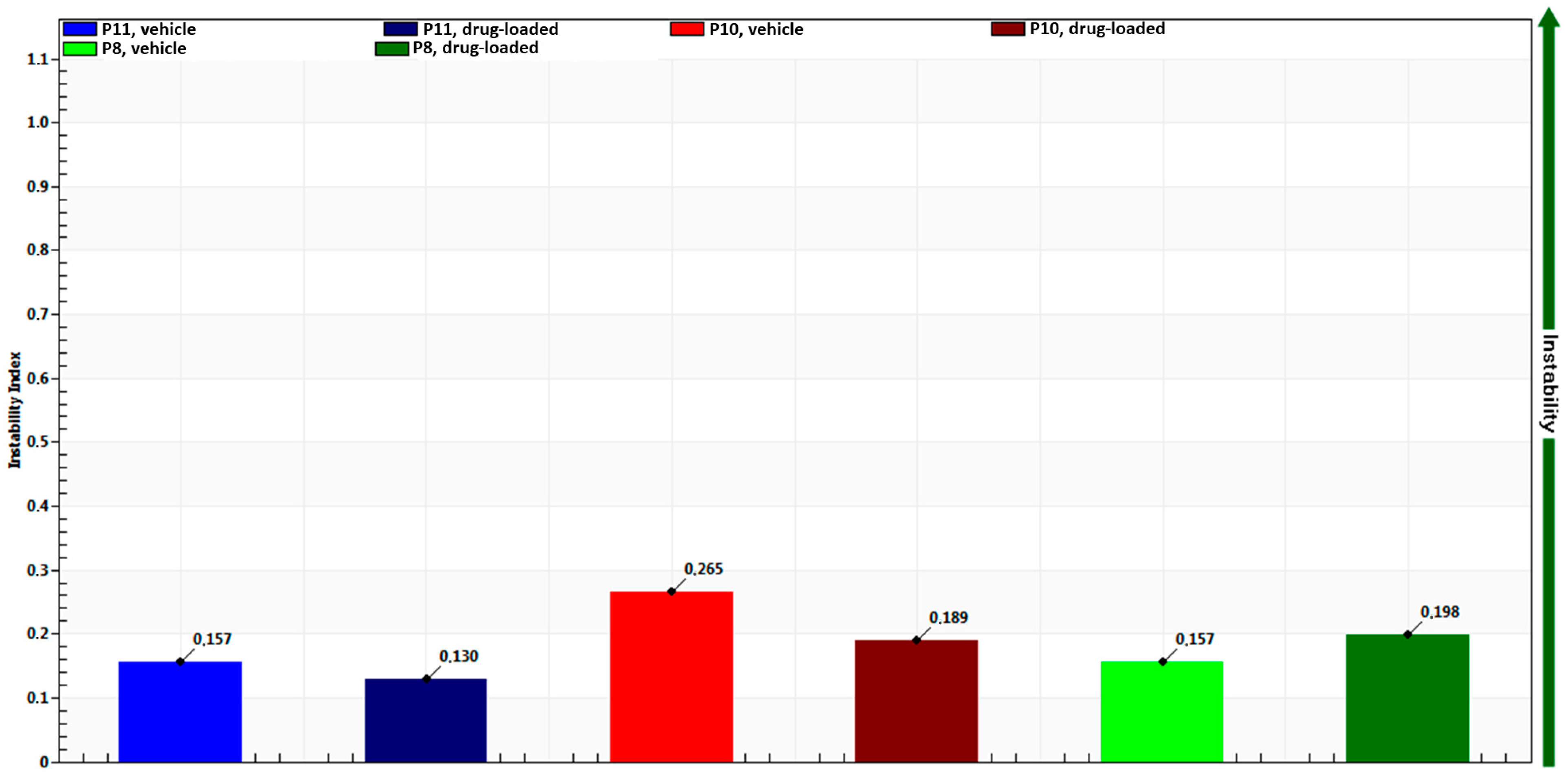
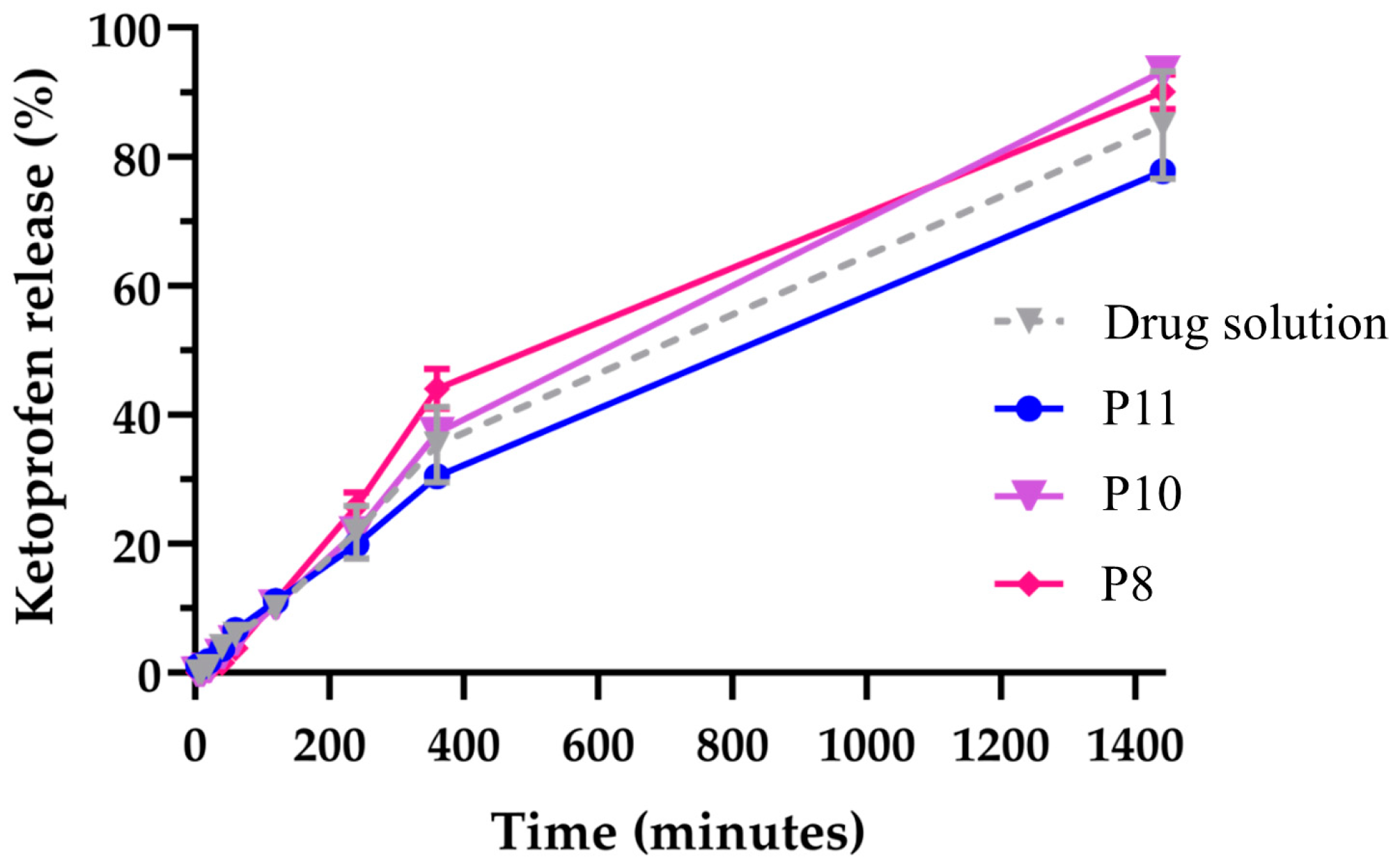
3. Results and Discussion
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hua, Y.; Dai, X.; Xu, Y.; Xing, G.; Liu, H.; Lu, T.; Chen, Y.; Zhang, Y. Drug Repositioning: Progress and Challenges in Drug Discovery for Various Diseases. Eur. J. Med. Chem. 2022, 234, 114239. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.S.; Prasad, A.; Pradeep, S.; Dharmashekar, C.; Achar, R.R.; Ekaterina, S.; Victor, S.; Amachawadi, R.G.; Prasad, S.K.; Pruthvish, R.; et al. Everything Old Is New Again: Drug Repurposing Approach for Non-Small Cell Lung Cancer Targeting MAPK Signaling Pathway. Front. Oncol. 2021, 11, 741326. [Google Scholar] [CrossRef] [PubMed]
- Turabi, K.S.; Deshmukh, A.; Paul, S.; Swami, D.; Siddiqui, S.; Kumar, U.; Naikar, S.; Devarajan, S.; Basu, S.; Paul, M.K.; et al. Drug Repurposing—An Emerging Strategy in Cancer Therapeutics. Naunyn-Schmiedeberg Arch. Pharmacol. 2022, 395, 1139–1158. [Google Scholar] [CrossRef]
- Fu, L.; Jin, W.; Zhang, J.; Zhu, L.; Lu, J.; Zhen, Y.; Zhang, L.; Ouyang, L.; Liu, B.; Yu, H. Repurposing Non-Oncology Small-Molecule Drugs to Improve Cancer Therapy: Current Situation and Future Directions. Acta Pharm. Sin. B 2022, 12, 532–557. [Google Scholar] [CrossRef] [PubMed]
- Lyne, S.B.; Yamini, B. An Alternative Pipeline for Glioblastoma Therapeutics: A Systematic Review of Drug Repurposing in Glioblastoma. Cancers 2021, 13, 1953. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Cervi, V.F.; Gehrcke, M.; da Silveira, E.F.; Azambuja, J.H.; Braganhol, E.; Sari, M.H.M.; Zborowski, V.A.; Nogueira, C.W.; Cruz, L. Ketoprofen-Loaded Pomegranate Seed Oil Nanoemulsion Stabilized by Pullulan: Selective Antiglioma Formulation for Intravenous Administration. Colloids Surf. B Biointerfaces 2015, 130, 272–277. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, E.F.; Chassot, J.M.; Teixeira, F.C.; Azambuja, J.H.; Debom, G.; Beira, F.T.; Del Pino, F.A.B.; Lourenço, A.; Horn, A.P.; Cruz, L.; et al. Ketoprofen-Loaded Polymeric Nanocapsules Selectively Inhibit Cancer Cell Growth in Vitro and in Preclinical Model of Glioblastoma Multiforme. Investig. New Drugs 2013, 31, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Javeed, A.; Ashraf, M.; Al-Zaubai, N.; Stewart, A.; Mukhtar, M.M. Non-Steroidal Anti-Inflammatory Drugs, Tumour Immunity and Immunotherapy. Pharmacol. Res. 2012, 66, 7–18. [Google Scholar] [CrossRef]
- Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in Cancer: A Review. J. Cell. Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef]
- Pu, D.; Yin, L.; Huang, L.; Qin, C.; Zhou, Y.; Wu, Q.; Li, Y.; Zhou, Q.; Li, L. Cyclooxygenase-2 Inhibitor: A Potential Combination Strategy With Immunotherapy in Cancer. Front. Oncol. 2021, 11, 637504. [Google Scholar] [CrossRef] [PubMed]
- Patra, I.; Naser, R.H.; Hussam, F.; Hameed, N.M.; Kadhim, M.M.; Ahmad, I.; Awadh, S.A.; Hamad, D.A.; Parra, R.M.R.; Mustafa, Y.F. Ketoprofen Suppresses Triple Negative Breast Cancer Cell Growth by Inducing Apoptosis and Inhibiting Autophagy. Mol. Biol. Rep. 2023, 50, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Q.; Li, L.; Chen, Y.; Cui, J.; Liu, M.; Zhang, N.; Liu, Z.; Han, J.; Wang, Z. Ketoprofen and Loxoprofen Platinum(IV) Complexes Displaying Antimetastatic Activities by Inducing DNA Damage, Inflammation Suppression, and Enhanced Immune Response. J. Med. Chem. 2021, 64, 17920–17935. [Google Scholar] [CrossRef] [PubMed]
- Sarheed, O.; Dibi, M.; Ramesh, K.V.R.N.S. Studies on the Effect of Oil and Surfactant on the Formation of Alginate-Based O/W Lidocaine Nanocarriers Using Nanoemulsion Template. Pharmaceutics 2020, 12, 1223. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, Properties and Applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Handa, M.; Ujjwal, R.R.; Vasdev, N.; Flora, S.J.S.; Shukla, R. Optimization of Surfactant- and Cosurfactant-Aided Pine Oil Nanoemulsions by Isothermal Low-Energy Methods for Anticholinesterase Activity. ACS Omega 2021, 6, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.C.; Santos, L.T.; Rodrigues, M.; Alves, G.; Santos, A.O. Intranasal Fosphenytoin: The Promise of Phosphate Esters in Nose-to-Brain Delivery of Poorly Soluble Drugs. Int. J. Pharm. 2021, 592, 120040. [Google Scholar] [CrossRef] [PubMed]
- Ketoprofen. Available online: https://go.drugbank.com/drugs/DB01009 (accessed on 15 August 2023).
- National Center for Biotechnology Information PubChem Compound Summary for CID 3825, Ketoprofen. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ketoprofen (accessed on 15 August 2023).
- Barhoum, A.; García-Betancourt, M.L.; Rahier, H.; Van Assche, G. Physicochemical Characterization of Nanomaterials: Polymorph, Composition, Wettability, and Thermal Stability. In Emerging Applications of Nanoparticles and Architecture Nanostructures; Elsevier: Amsterdam, The Netherlands, 2018; pp. 255–278. [Google Scholar]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 697, pp. 63–70. [Google Scholar]
- Laxmi, M.; Bhardwaj, A.; Mehta, S.; Mehta, A. Development and Characterization of Nanoemulsion as Carrier for the Enhancement of Bioavailability of Artemether. Artif. Cells Nanomed. Biotechnol. 2015, 43, 334–344. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, P.C.; Correia, M.; Veiga, F.; Paiva-Santos, A.C. Anti-Inflammatory Drug Repurposing for Intranasal Delivery: Ketoprofen Nanoemulgel Development for the Treatment of Glioma. Eng. Proc. 2023, 56, 20. https://doi.org/10.3390/ASEC2023-15345
Pires PC, Correia M, Veiga F, Paiva-Santos AC. Anti-Inflammatory Drug Repurposing for Intranasal Delivery: Ketoprofen Nanoemulgel Development for the Treatment of Glioma. Engineering Proceedings. 2023; 56(1):20. https://doi.org/10.3390/ASEC2023-15345
Chicago/Turabian StylePires, Patrícia C., Mafalda Correia, Francisco Veiga, and Ana Cláudia Paiva-Santos. 2023. "Anti-Inflammatory Drug Repurposing for Intranasal Delivery: Ketoprofen Nanoemulgel Development for the Treatment of Glioma" Engineering Proceedings 56, no. 1: 20. https://doi.org/10.3390/ASEC2023-15345
APA StylePires, P. C., Correia, M., Veiga, F., & Paiva-Santos, A. C. (2023). Anti-Inflammatory Drug Repurposing for Intranasal Delivery: Ketoprofen Nanoemulgel Development for the Treatment of Glioma. Engineering Proceedings, 56(1), 20. https://doi.org/10.3390/ASEC2023-15345







