Analysis of Nanodrug Delivery in Blood Flowing through Blood Vessels Using Machine Learning Models †
Abstract
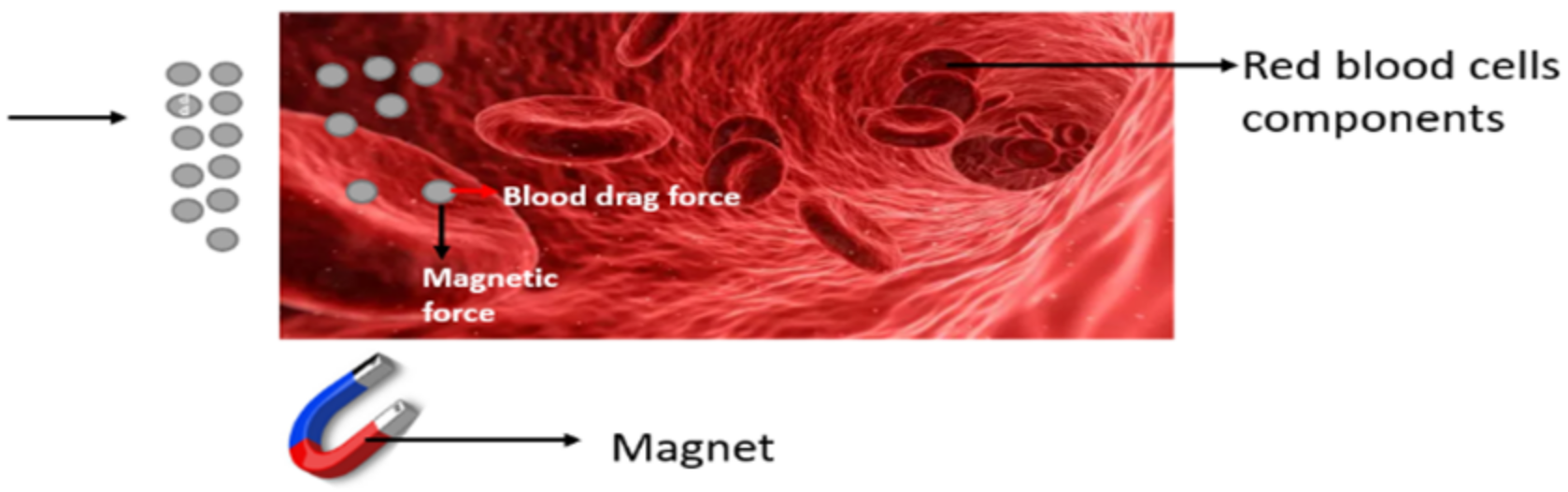
:1. Introduction
2. Formulation of the Problem
- Expressing (1) and (2) component-wise we obtain:
3. Solution for the Newtonian Model
4. Modelling of the Concentration of Nano Drug Particle
Finite Difference Method
- Dropping the hats in (26)–(29) and defining:
5. Description of the Data
6. Machine Learning Modelling
6.1. Multilayer Perceptron (MLP)
6.2. Decision Tree
6.3. Gradient Boosting Regressor
6.4. XG (Extreme Gradient) Boost Regressor
6.5. CatBoost Regressor
7. Statistical Accuracy Metrics
8. Results and Discussion
9. Conclusions
10. Future Work and Scope
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef]
- Grief, A.D.; Richardson, G. Mathematical modelling of magnetically targeted drug delivery. J. Magn. Magn. Mater. 2005, 293, 455–463. [Google Scholar] [CrossRef]
- Rukshin, I.; Mohrenweiser, J.; Yue, P.; Afkhami, S. Modeling superparamagnetic particles in blood flow for applications in magnetic drug targeting. Fluids 2017, 2, 29. [Google Scholar] [CrossRef]
- Nadeem, S.; Ijaz, S. Theoretical analysis of metallic nanoparticles on blood flow through stenosed artery with permeable walls. Phys. Lett. A 2015, 379, 542–554. [Google Scholar] [CrossRef]
- Nacev, A.; Beni, C.; Bruno, O.; Shapiro, B. The behaviors of ferromagnetic nano-particles in and around blood vessels under applied magnetic fields. J. Magn. Magn. Mater. 2011, 323, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hamdeh, N.H.; Bantan, R.A.; Aalizadeh, F.; Alimoradi, A. Controlled drug delivery using the magnetic nanoparticles in non-Newtonian blood vessels. Alex. Eng. J. 2020, 59, 4049–4062. [Google Scholar] [CrossRef]
- Bhatti, M.M.; Sait, S.M.; Ellahi, R. Magnetic nanoparticles for drug delivery through tapered stenosed artery with blood based non-newtonian fluid. Pharmaceuticals 2022, 15, 1352. [Google Scholar] [CrossRef] [PubMed]
- Tiam Kapen, P.; Njingang Ketchate, C.G.; Fokwa, D.; Tchuen, G. Linear stability analysis of non-Newtonian blood flow with magnetic nanoparticles: Application to controlled drug delivery. Int. J. Numer. Methods Heat Fluid Flow 2022, 32, 714–739. [Google Scholar] [CrossRef]
- Ponalagusamy, R.; Selvi, R.T.; Padma, R. Modeling of pulsatile EMHD flow of non-Newtonian blood with magnetic particles in a tapered stenosed tube: A comparative study of actual and approximated drag force: EMHD flow of Bingham fluid. Eur. Phys. J. Plus 2022, 137, 230. [Google Scholar] [CrossRef]
- Aryan, H.; Beigzadeh, B.; Siavashi, M. Euler-Lagrange numerical simulation of improved magnetic drug delivery in a three-dimensional CT-based carotid artery bifurcation. Comput. Methods Programs Biomed. 2022, 219, 106778. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Vasu, B.; Anwar Bég, O.; Gorla, R.S.; Kadir, A. Computational fluid dynamic simulation of two-fluid non-Newtonian nanohemodynamics through a diseased artery with a stenosis and aneurysm. Comput. Methods Biomech. Biomed. Eng. 2020, 23, 345–371. [Google Scholar] [CrossRef] [PubMed]
- Vasu, B.; Dubey, A.; Bég, O.A.; Gorla, R.S.R. Micropolar pulsatile blood flow conveying nanoparticles in a stenotic tapered artery: Non-Newtonian pharmacodynamic simulation. Comput. Biol. Med. 2020, 126, 104025. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, J.; Vasu, B.; Bég, O.A.; Gorla, R.S.R.; Kameswaran, P.K. Computational simulation of rheological blood flow containing hybrid nanoparticles in an inclined catheterized artery with stenotic, aneurysmal and slip effects. Comput. Biol. Med. 2021, 139, 105009. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, J.; Vasu, B.; Bég, O.A.; Mounika, B.R.; Gorla, R.S.R. Numerical simulation of the transport of nanoparticles as drug carriers in hydromagnetic blood flow through a diseased artery with vessel wall permeability and rheological effects. Microvasc. Res. 2022, 139, 104241. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.A.; Mohapatra, S.; Panonnummal, R. Formulation and evaluation of magnesium sulphate nanoparticles for improved CNS penetrability. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Misra, J.C.; Shit, G.C. Role of slip velocity in blood flow through stenosed arteries: A non-Newtonian model. J. Mech. Med. Biol. 2007, 7, 337–353. [Google Scholar] [CrossRef]
- Nadesh, R.; Narayanan, D.; Pr, S.; Vadakumpully, S.; Mony, U.; Koyakkutty, M.; Nair, S.V.; Menon, D. Hematotoxicological analysis of surface-modified and-unmodified chitosan nanoparticles. J. Biomed. Mater. Res. Part A 2013, 101, 2957–2966. [Google Scholar] [CrossRef] [PubMed]
- Asha, K.N.; Srivastava, N. Geometry of stenosis and its effects on the blood flow through an artery-A theoretical study. In Proceedings of the Essence of Mathematics in Engineering Applications (EMEA-2020), Guntur, India, 2–3 December 2020. [Google Scholar]
- Fanelli, C.; Kaouri, K.; Phillips, T.N.; Myers, T.G.; Font, F. Magnetic nanodrug delivery in non-Newtonian blood flows. Microfluid. Nanofluidics 2022, 26, 74. [Google Scholar] [CrossRef]
- Selladurai, S.J.; Srivastava, N.; Pati, P.B. Machine learning analysis of shear stress over a symmetrically stenosed arterial wall under the impact of magnetic field. In Proceedings of the 2023 2nd International Conference on Vision Towards Emerging Trends in Communication and Networking Technologies (ViTECoN), Vellore, India, 5–6 May 2023; IEEE: Piscataway, NJ, USA, 2023. [Google Scholar]


| Machine Learning Models | RMSE | R2 | AARD | STD |
|---|---|---|---|---|
| CatBoost | Training data: 0.0047 | Training data: 0.99 | Training data: −3.40 | Training data: −4.1 |
| Regressor | Testing data: 0.472 | Testing data: 0.989 | Testing data: 222.52 | Testing data: 4.610 |
| Decision | Training data: 0.303 | Training data: 0.994 | Training data: −29.4 | Training data: 4.09 |
| Tree | Testing data: 0.461 | Testing data: 0.990 | Testing data: 382.0 | Testing data: 4.70 |
| Gradient Boosting | Training data: 0.22 | Training data: 0.99 | Training data: −8.8 | Training data: 3.8 |
| Regressor | Testing data: 0.476 | Testing data: 0.980 | Testing data: 526.0 | Testing data: 4.30 |
| XG Boost | Training data: 0.34 | Training data: 0.99 | Training data: −5.3 | Training data: 3.8 |
| Testing data: 0.640 | Testing data: 0.989 | Testing data: 155.4 | Testing data: 4.35 | |
| Multiple-Layer | Training data: 0.436 | Training data: 0.988 | Training data: −30.3 | Training data: −3.97 |
| Perceptron | Testing data: 0.410 | Testing data: 0.992 | Testing data: 500.59 | Testing data: 4.620 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selladurai, S.J.; Srivastava, N.; Sarris, I.E. Analysis of Nanodrug Delivery in Blood Flowing through Blood Vessels Using Machine Learning Models. Eng. Proc. 2023, 50, 8. https://doi.org/10.3390/engproc2023050008
Selladurai SJ, Srivastava N, Sarris IE. Analysis of Nanodrug Delivery in Blood Flowing through Blood Vessels Using Machine Learning Models. Engineering Proceedings. 2023; 50(1):8. https://doi.org/10.3390/engproc2023050008
Chicago/Turabian StyleSelladurai, Spurthi Joanna, Neetu Srivastava, and Ioannis E. Sarris. 2023. "Analysis of Nanodrug Delivery in Blood Flowing through Blood Vessels Using Machine Learning Models" Engineering Proceedings 50, no. 1: 8. https://doi.org/10.3390/engproc2023050008
APA StyleSelladurai, S. J., Srivastava, N., & Sarris, I. E. (2023). Analysis of Nanodrug Delivery in Blood Flowing through Blood Vessels Using Machine Learning Models. Engineering Proceedings, 50(1), 8. https://doi.org/10.3390/engproc2023050008







