Abstract
Magnetite nanoparticles (MNPs) are a preferable material for different bioassays because of their quite low toxicity both for cells and for mammals, and they have a big variety of surface functionalization approaches. We have synthesized MNPs via a simple and convenient co-precipitation method with preliminary filtration of FeCl2 and FeCl3 solution, under argon atmosphere and non-magnetic stirring. MNPs were citrate-stabilized and then modified stage by stage with tetraethoxysilane (TEOS), (3-Aminopropyl)triethoxysilane (APTES) and acylated with succinic anhydride, resulting in carboxylated MNPs. Carboxylated MNPs were covalently bounded with folic acid antibody (FA-1) via 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC). MNP-EDC-FA-1 were passed through a test-stripe with the line consisting of folic acid–gelatin conjugate. The conjugation of MNP-EDC-FA-1 with folic acid was observed visually, and the magnetic signal distribution was scanned through the test-stripe with the magnetic particle quantification technique (MPQ) developed earlier. Visually, the line with folic acid–gelatin conjugate on the test-stripe turned dark, with color intensity strongly depending on the MNP-EDC-FA-1 concentration. MPQ has shown that the great majority of MNP-EDC-FA-1 was bound with the acid–gelatin conjugate. The MPQ technique allowed quantification down to 5 ng of MNP-EDC-FA-1 in this experiment with MNPs synthesized, with a strong peak at the acid–conjugate line.
1. Introduction
Magnetite nanoparticles (MNPs) are a preferable material for different bioassays [1] because of their relatively low toxicity for both cells [2] and for mammals [3], and they have a big variety of surface functionalization approaches [4,5]. Superparamagnetic behavior provides an application of MNPs as magnetic labels for both cells [6] and molecules [7]. A combination of MNPs optical properties at visible range, coupled with its magnetic properties, has led to the fundament of a magnetometric lateral flow immunoassay on test-stripes for rapid and sensitive qualitative and quantitative analysis of different biomolecules, for which the magnetic particle quantification (MPQ) technique was developed earlier [8,9].
2. Methods
We have synthesized MNPs via a simple and convenient co-precipitation method (Figure 1). Briefly, FeCl2 and FeCl3 were dissolved in degassed water in a stoichiometric rate and filtered in order to exclude hydroxy- and oxychlorides that may act as undesirable and big crystallization centers due to their low solubility. The synthesis of MNPs was carried out by adding NaOH in a degassed water solution to the mixture of FeCl2 and FeCl3, and stirred non-magnetically in order to minimize the formation of non-spherical structures under an argon atmosphere to prevent the MNPs from oxidation. MNPs were washed and stabilized with sodium citrate. Citrate-stabilized MNPs (MNP-cit) were modified stage by stage with tetraethoxysilane (TEOS), (3-Aminopropyl)triethoxysilane (APTES), resulting in aminated MNPs (MNP-NH2), and acylated with succinic anhydride, resulting in carboxylated MNPs (MNP-COOH). Carboxylated MNPs were covalently bounded with a folic acid antibody (FA-1) via 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) by incubation of carboxylated MNPs with EDC, washing, and then the consequent incubation with FA-1. MNP-EDC-FA-1 suspension was mixed with albumin buffer solution in order to block the unreacted EDC and to simulate blood serum media. Porous test-stripes with folic acid–gelatin conjugates were put into MNP-EDC-FA-1 suspension and left for 15 min. Then, the test-stripes were scanned with an MPQ-scanner in order to measure their magnetic signal distribution.
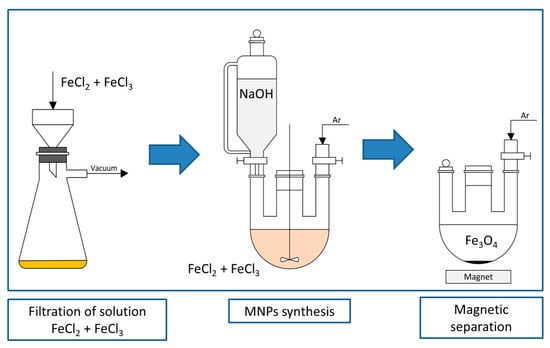
Figure 1.
Synthesis of MNP via co-precipitation method.
3. Results and Discussion
The suspension of synthesized nanoparticles was of a dark, black color. The magnetic response was strong. XRD (Figure 2) has shown that synthesized nanoparticles consisted of pure magnetite, with a crystallite size of about 12 nm, according to the Scherrer equation. Sodium citrate dihydrate peaks were observed on the diffractogram of MNP-cit synthesized in an air atmosphere, which may indicate its excess on the MNP surface due to the insufficient washing of MNPs. Peaks corresponding to the magnetite are better pronounced when MNPs are synthesized in an Ar atmosphere.
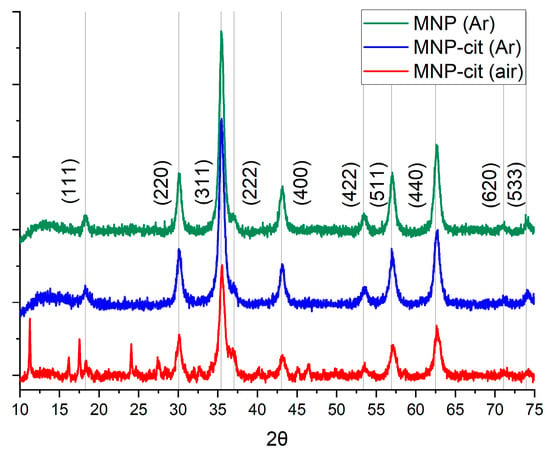
Figure 2.
XRD of MNPs synthesized: MNP (Ar)—pristine MNPs synthesized in Ar atmosphere, MNP-cit (Ar)—citrate-stabilized MNPs synthesized in an Ar atmosphere, MNP-cit (air)—citrate-stabilized MNPs synthesized in air atmosphere.
Hydrodynamic radii (Figure 3) of pristine MNPs agglomerates were about 380 nm and decreased to 136 nm after modification with sodium citrate. Carboxylation caused no sufficient resultant change in MNPs agglomerates’ size. ζ-potential changed from neutral to −48 ± 7 mV after modification with sodium citrate, and alongside a size decrease that indicated the stabilization of the suspension, resulted in +25 ± 7 mV after amination with APTES and −25 ± 10 mV after acylation, indicating that carboxylation was successful.
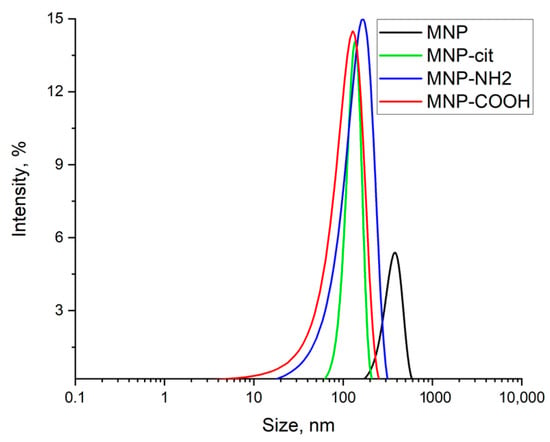
Figure 3.
Hydrodynamic radii of particles in MNP suspensions: MNP—pristine MNPs, MNP-cit—citrate-stabilized MNPS, MNP-NH2—aminated MNPs, MNP-COOH—carboxylated MNPs.
The conjugation of MNP-EDC-FA-1 with folic acid was observed visually, and the magnetic signal was scanned through the test-stripe by the MPQ-magnetometer (Figure 4). Visually, the line with folic acid–gelatin conjugate on the test-stripe turned dark, with its color intensity strongly dependent on the MNP-EDC-FA-1 concentration. MPQ showed that the great majority of MNP-EDC-FA-1 was bound with the acid–gelatin conjugate. The MPQ technique allowed quantification down to 5 ng of MNP-EDC-FA-1 in this experiment with MNPs synthesized, with strong peak at the acid–gelatin conjugate line.
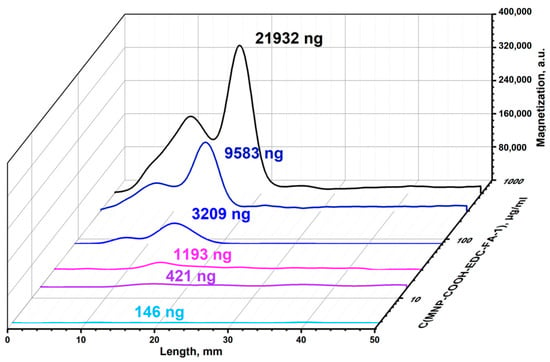
Figure 4.
Magnetograms of test stripes with MNP-COOH-EDC-FA-1 against FA-gelatin conjugates.
4. Conclusions
The synthesis of MNPs in an Ar atmosphere allows the obtaining of MNPs without any other phases but magnetite. MNPs may serve both as a platform for immobilization of antibodies and a magnetic label for them. MPQ may be quite a precise tool for detection and quantitative analysis of MNPs, so the magnetometric lateral flow immunoassay is possible to be utilized, for example, to quantify the interaction between antibody and antigen.
Author Contributions
Conceptualization, A.V.S. and S.L.Z.; investigation, M.O.Z.; methodology, A.V.S. and S.L.Z.; project administration, A.V.O. and P.I.N.; resources, P.I.N.; supervision, A.V.S.; writing, M.O.Z. and A.V.S.; writing—review and editing, M.O.Z. and A.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This multidisciplinary research was supported by the grant of Russian Science Foundation No. 19-73-10205.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We are grateful to A.A. Alexandrov (Prokhorov General Physics Institute of the Russian Academy of Sciences) for XRD measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fleming, C.L.; Golzan, M.; Gunawan, C.; McGrath, K.C. Systematic and Bibliometric Analysis of Magnetite Nanoparticles and Their Applications in (Biomedical) Research. Glob. Chall. 2022, 7, 2200009. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Simchi, A.; Milani, A.; Stroeve, P. Cell toxicity of superparamagnetic iron oxide nanoparticles. J. Colloid Interface Sci. 2009, 336, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.M.V.; de Souza, V.B.; Panunto, P.C.; Nicolosi, J.S.; da Silva, E.D.N.; Cadore, S.; Londono, O.M.; Muraca, D.; Tancredi, P.; de Brot, M.; et al. In vitro and in vivo acute toxicity of a novel citrate-coated magnetite nanoparticle. PLoS ONE 2022, 17, e0277396. [Google Scholar] [CrossRef]
- Popescu, R.C.; Andronescu, E.; Vasile, B.S. Recent advances in magnetite nanoparticle functionalization for nanomedicine. Nanomaterials 2019, 9, 1791. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.-V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Kamihira, M. Tissue Engineering Using Magnetite Nanoparticles, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 104. [Google Scholar] [CrossRef]
- Mabarroh, N.; Alfansuri, T.; Wibowo, N.A.; Istiqomah, N.I.; Tumbelaka, R.M.; Suharyadi, E. Detection of green-synthesized magnetite nanoparticles using spin-valve GMR-based sensor and their potential as magnetic labels. J. Magn. Magn. Mater. 2022, 560, 169645. [Google Scholar] [CrossRef]
- Pushkarev, A.V.; Orlov, A.V.; Znoyko, S.L.; Bragina, V.A.; Nikitin, P.I. Rapid and easy-to-use method for accurate characterization of target binding and kinetics of magnetic particle bioconjugates for biosensing. Sensors 2021, 21, 2802. [Google Scholar] [CrossRef]
- Guteneva, N.V.; Znoyko, S.L.; Orlov, A.V.; Nikitin, M.P.; Nikitin, P.I. Rapid lateral flow assays based on the quantification of magnetic nanoparticle labels for multiplexed immunodetection of small molecules: Application to the determination of drugs of abuse. Microchim. Acta 2019, 186, 621. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).