Abstract
Conjugated polymers (CPs) are an intriguing material with which to build fluorescent Cr2O72− sensors with excellent sensitivity, but they often lack specific recognition groups. In this study, several typical amino acids with N and O atom-identifying groups were incorporated into fluorene, and then six polyfluorene derivatives were synthesized using electrochemical polymerization. Compared to other cations and anions, all of these amino acid-functionalized polyfluorenes have good selectivity towards Cr2O72− and enable ultra-trace responses with detection thresholds at pM or even fM level.
1. Introduction
The development of modern industry has caused a large amount of heavy metals to be released, causing transitional accumulation of heavy metals in the soil and expanding the area of contamination, thereby affecting the growth of crops and lowering the quality of products. Cadmium ion is more frequently acknowledged as an internationally recognized carcinogen than other heavy metal ions [1]. For example, once its content exceeds the standard in water (existing in the form of Cr2O72−), it will be enriched in crops and passed onto humans through the food chain, which will cause various diseases [2,3]. Therefore, it is necessary to detect Cr2O72− quickly, easily, and accurately.
Among the various methods for the detection of Cr2O72−, fluorescence analysis has attracted significant attention due to its high sensitivity, simplicity, and rich output signal [4]. Conjugated polymers (CPs) have high molar absorption coefficients and strong light absorption abilities, and are good light energy harvesters [5]. Moreover, these molecules have a unique “molecular line effect” that can amplify the fluorescence response signal hundreds or thousands of times, which makes them stand out in the fields of biomonitoring and environmental analysis [6,7]. Unfortunately, there have been few reports on the specific identification of Cr2O72− relying on CPs-based fluorescent materials.
Fluorene is a classical blue luminescent polymer material that not only has a high fluorescence quantum yield and good optical stability, but can also further improve its photoelectric performance by introducing amino acid groups containing N and O atoms in the side chain [8,9]. Amino acids containing N and O atoms are characterized by high ion-binding ability, good selectivity, and a strong pH response, which give them certain advantages in the development of efficient and sensitive ion sensors [10,11]. Therefore, our group proposed a design strategy, selecting fluorene as the fluorescence group and introducing amino acid moieties at the C9 position as recognition groups. Then, six polyfluorene fluorescent derivatives were successfully fabricated via electrochemical polymerization to construct fluorescence sensors for Cr2O72− detection [2,3,10,11].
2. Materials and Methods
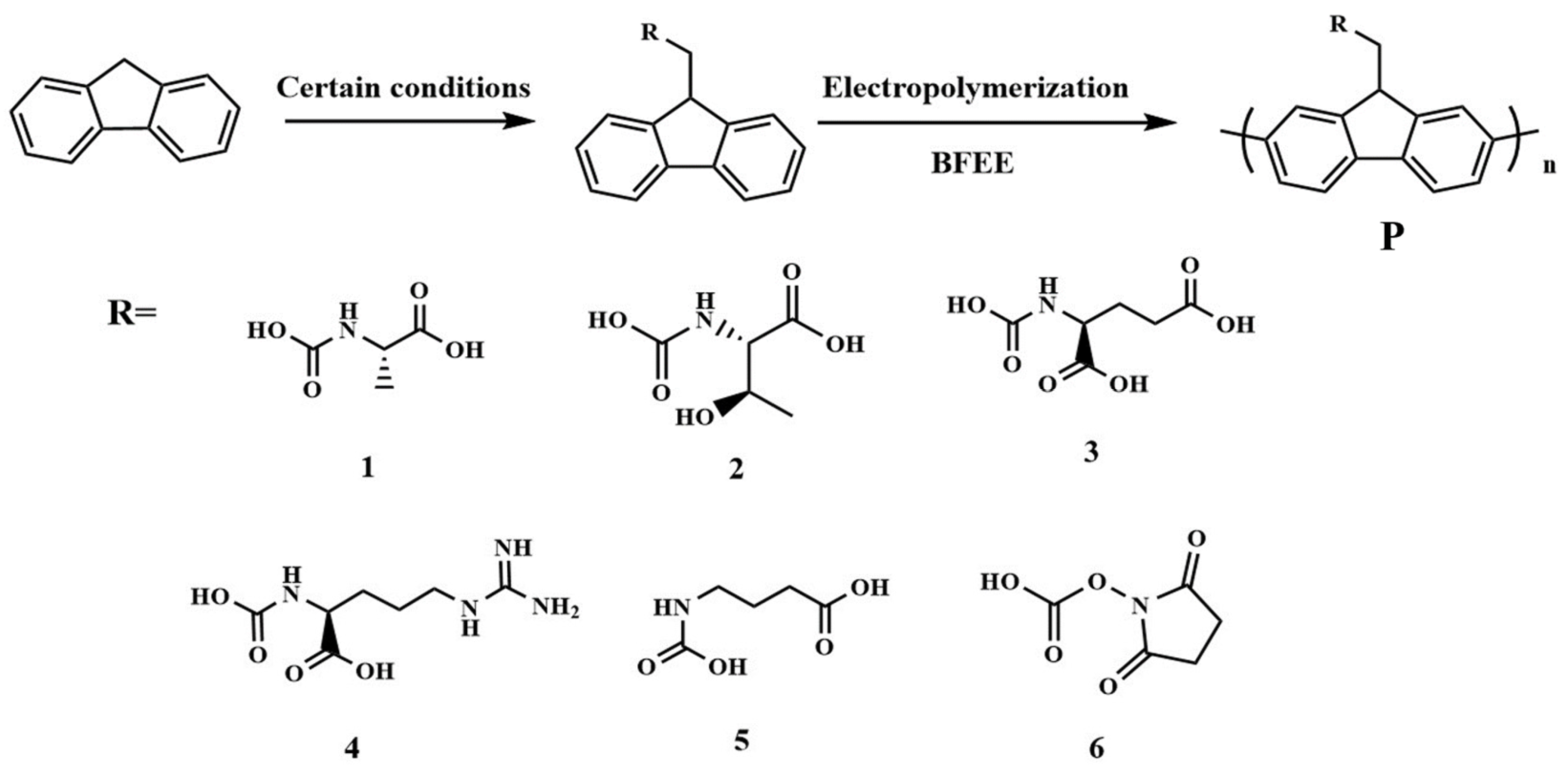
Six short chains modified with N and O atoms as recognition groups were introduced into fluorene to obtain six monomers (Fmoc-Arg-OH, Fmoc-Glu-OH, Fmoc-GABA-OH, Fmoc-Osu, Fmoc-Ala-OH and Fmoc-Thr-OH) before electrochemical polymerization in the boron trifluoride ethyl ether (BFEE) system (Figure 1). Considering that six monomers cannot be electrochemically polymerized in common organic solvents such as tetrahydrofuran (THF), N,N-dimethylformamide (DMF), and dimethyl sulfoxide (DMSO) containing supporting electrolytes, an attempt was made to add trifluoroacetic acid (TFA) into BFEE as the electrolyte solution. On one hand, BFEE, as a Lewis acid, has a catalytic effect on the electropolymerization of aromatic compounds [2,3,6,11]. On the other hand, the addition of TFA to BFEE leads to the formation of complexes between the monomer and TFA, which reduces the resonance of the aromatic ring and improves the stability of the radical ion while increasing the electrical conductivity [2,3,10,11].
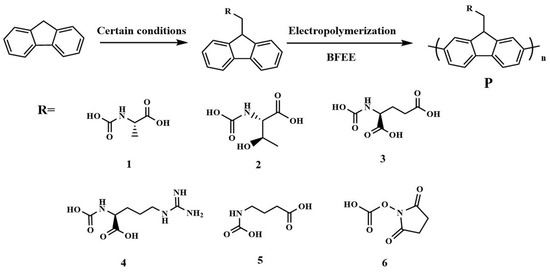
Figure 1.
Electrochemical polymerization of six fluorenyl derivatives. (1, Fmoc-Ala-OH; 2, Fmoc-Thr-OH; 3, Fmoc-Arg-OH; 4, Fmoc-Glu-OH; 5, Fmoc-GABA-OH; 6, Fmoc-Osu).
3. Results and Discussion
3.1. Selectivity of Polyfluorene Derivatives towards Cr2O72−
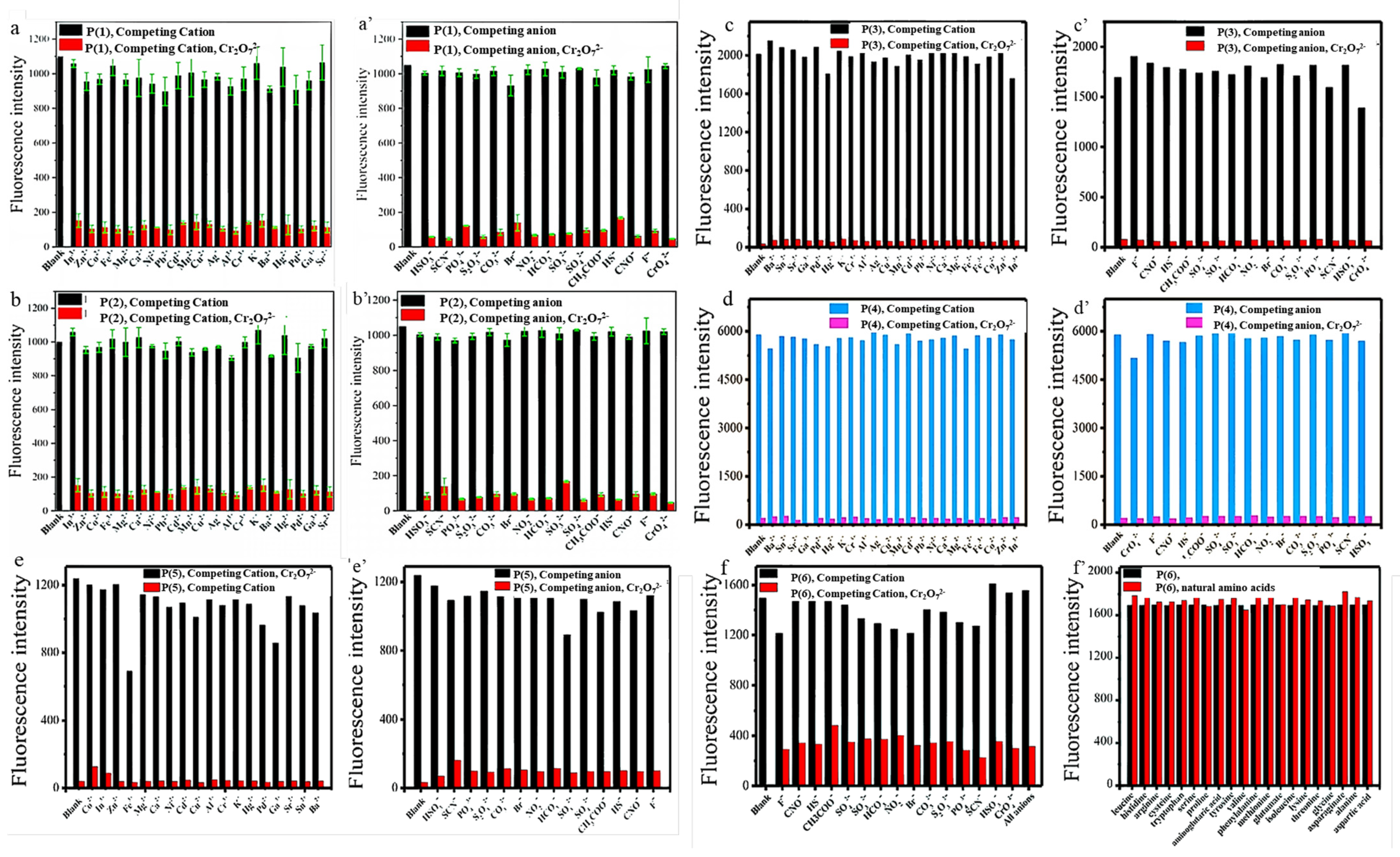
To explore the recognition capability of Cr2O72−, selectivity experiments with six polyfluorenes for common anions and cations were carried out. It has been experimentally shown that only Cr2O72− could quench the fluorescence of six polymers, which indicated these molecules could specifically recognize Cr2O72−. It is speculated that the N and O atoms may interact with Cr2O72−, which caused the aggregation of the fluorene skeleton and resulted in its fluorescence quenching [2,3,10,11]. All the polymers also exhibited good selectivity towards Cr2O72−, which was not interfered with by other anions and cations. Besides introducing N and O atoms for chelating Cr2O72−, another reason for using amino acid groups was to exclude the interference of natural amino acids in agricultural detection. Such good anti-interference ability is shown in Figure 2 and Figure 3.
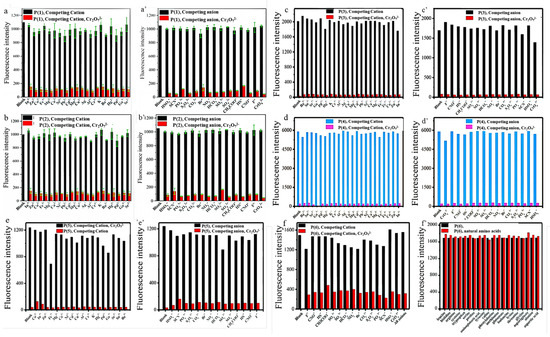
Figure 2.
Fluorescence response of P (1) (a,a’), P (2) (b,b’), P (3) (c,c’), P (4) (d,d’), P (5) (e,e’) and P (6) (f,f’) in the mixed DMSO/PBS (1:1000, v/v) containing various ions. The black and blue bars represent the addition of the competing ions to a solution of six polymers. The red and pink bars represent the change of the emission that occurs upon the subsequent addition of Cr2O72− to the above solution [2,3,10,11].
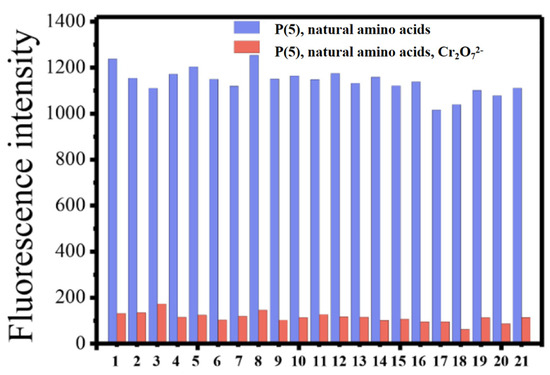
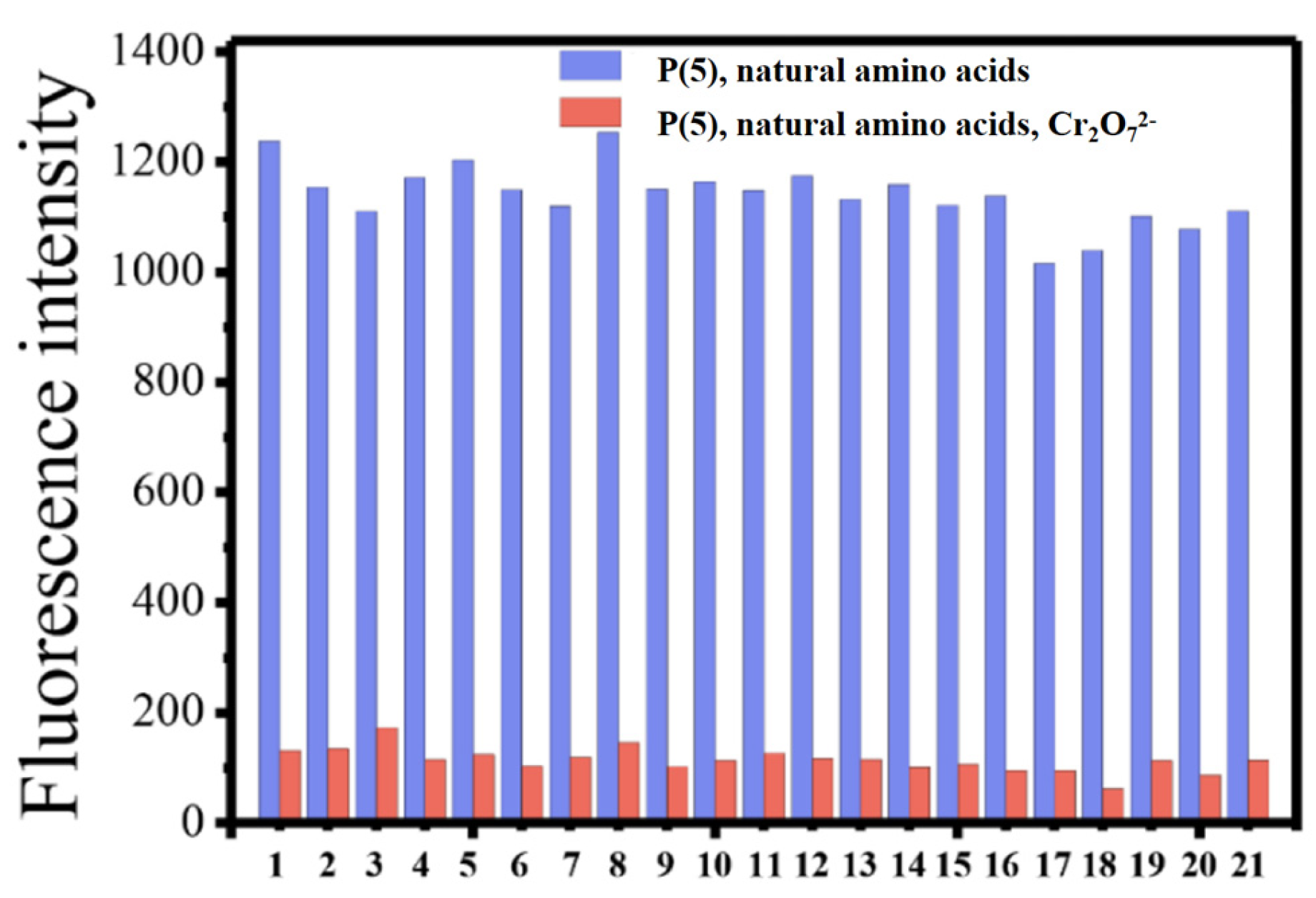
Figure 3.
Fluorescence intensity of P(5) upon the addition of 0.1 mM Cr2O72− to DMSO-PBS (v/v = 1:500) solution containing 0.1 mM natural amino acids. The abscissa is from left to right: 1. Blank, 2. Cysteine, 3. Arginine, 4. Histidine, 5. Isoleucine, 6. Asparagine, 7. Glycine, 8. Alanine, 9. Proline, 10. Glutamine, 11. Serine, 12. Aspartic acid, 13. Valine, 14. Lysine, 15. Glutamic acid, 16. Threonine, 17. Tyrosine, 18. Methionine, 19. Leucine, 20. Phenylalanine, 21. Tryptophan. (Ex = 334 nm).
3.2. Sensitivity Test of Fluorene and Polyfluorene Derivatives towards Cr2O72−
According to the linear relationship tests between the fluorescence intensity of fluorenes and polyfluorene derivatives in DMSO-EtOH solutions and the analyzed Cr2O72− concentrations, we further explored their sensitivity, and the linear relationship between fluorescence intensity and Cr2O72− concentration was studied. As shown in Table 1, monomers 1 and 2 have sensitivity to Cr2O72− at the nM level, while the other four monomers only achieve μM. When they were prepared as polymers, their detection sensitivity greatly improved, reaching up to pM and even fM (Table 1), which verifies that the molecular wire effect of polymers can greatly improve the sensitivity of the detection of Cr2O72−, which further indicates that this type of amino acid-functionalized polyfluorene fluorescence material has the ability to carry out ultra-trace detection of Cr2O72− [2,3,10,11].

Table 1.
Limit of detection (LOD) for monomers and polymers.
4. Conclusions
In sum, this paper summarizes a design strategy that involves the construction of a series of amino acid-functionalized polyfluorenes as fluorescent Cr2O72− sensors with high selectivity and sensitivity. Thanks to the side-chain groups decorated with N and O atoms, the obtained six monomers and their polymers were not interfered with by common cations, anions, or natural amino acids, and could achieve specific recognition of Cr2O72−. In comparison, the polymers showed a much higher sensitivity to LODs at the pM and fM levels. This work provides an effective method for the design of high-efficiency fluorescent sensors for Cr2O72− even in complex environmental systems.
Author Contributions
Conceptualization, G.Z.; methodology, W.Z.; software, W.Z.; validation, W.Z. and G.Z.; formal analysis, S.C.; investigation, H.L., Y.L. and L.C.; resources, G.Z.; data curation, W.Z., H.L., Y.L., L.S. and L.C.; writing—original draft preparation, W.Z.; writing—review and editing, S.C. and G.Z.; visualization, W.Z.; supervision, G.Z. and S.C.; project administration, G.Z.; funding acquisition, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful to the National Natural Science Foundation (5210031010), the Jiangxi Provincial Natural Science Foundation of (GJJ2201301, 20212BAB203012), and the Scientific Fund of Jiangxi Science & Technology Normal University (2022QNBJRC004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kapoor, R.T.; Mfarrej, M.F.B.; Alam, P.; Rinklebe, J.; Ahmad, P. Accumulation of chromium in plants and its repercussion in animals and humans. Environ. Pollut. 2022, 301, 119044. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Duan, X.; Li, H.; Zou, L.; Liu, G.; Liu, F.; Zhang, G.; Xu, J. Dual effect of aminobutyric acid group and “molecular wire effect” of conjugated polymer enables ultra-trace detection of Cr2O72− in fruits. Microchem. J. 2022, 178, 107426. [Google Scholar] [CrossRef]
- Li, F.; Duan, X.; Hu, S.; Zhang, L.; Shen, L.; Liu, F.; Li, H.; Zhang, G.; Xu, J. Ultra-sensitive detection of Cr2O72− in farmland achieved by an electrosynthesized fluorescent poly (Fmoc-succinimide). Dyes Pigment. 2021, 193, 109568. [Google Scholar] [CrossRef]
- Li, C.; Numata, M.; Takeuchi, M.; Shinkai, S. A sensitive colorimetric and fluorescent probe based on a polythiophene derivative for the detection of ATP. Angew. Chem. Int. Ed. 2005, 44, 6371–6374. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.W.; Kim, T.Y.; Woo, M.A. Trends in sensor development toward next-generation point-of-care testing for mercury. Biosens. Bioelectron. 2021, 183, 113228. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Wu, X.; Zhang, Y.; Wu, D.; Meng, L.; Chen, Y.; Tang, X. Recent applications of hydrogels in food safety sensing: Role of hydrogels. Trends Food Sci. Tech. 2022, 129, 244–257. [Google Scholar] [CrossRef]
- Li, R.; Liang, F.; Hu, X.; Bian, H.; Deng, C.; Seidi, F.; Liu, Y. A versatile cellulose nanocrystal-carbon dots architecture: Preparation and environmental/biological applications. Carbohyd. Polym. 2022, 298, 120073. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, A.K.; Prasad, K.M.; Trivedi, V.; Iyer, P.K. Interaction of heme proteins with anionic polyfluorene: Insights into physiological effects, folding events, and inhibition activity. ACS Appl. Mater. Interfaces 2012, 4, 6371–6377. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, A.K.; Saikia, G.; Iyer, P.K. Aqueous polyfluorene probe for the detection and estimation of Fe3+ and inorganic phosphate in blood serum. J. Mater. Chem. 2011, 21, 2502–2507. [Google Scholar] [CrossRef]
- Li, F.; Zhang, G.; Zou, L.; Zhang, X.; Liu, F.; Li, H.; Xu, J.; Duan, X. Amino acid groups enable electrosynthesized polyfluorenes to specifically recognize Cr2O72−. ACS Appl. Polym. Mater. 2022, 4, 815–821. [Google Scholar] [CrossRef]
- Li, H.; Li, F.; Liu, F.; Chen, X.; Xu, W.; Shen, L.; Xu, J.; Yang, R.; Zhang, G. High-quality conjugated polymers achieving ultra-trace detection of Cr2O72− in agricultural products. Molecules 2022, 27, 4294. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).