1. Introduction
A literature review shows that many organic reagents have been proposed for the determination of iron and other metals, but only a few are highly sensitive and selective. Essentially, the sensitive ones have low selectivity, and the selective ones have low sensitivity. Therefore, the development of new detection methods for iron detection with high sensitivity and selectivity is an urgent problem and the main goal of our scientific research [
1,
2].
Currently, photometric and spectrophotometric methods are widely used to determine the amount of a substance. In this work, the works of the photometric and spectrophotometric determination of iron(III) are considered.
Photometric methods are widely used in the determination of iron, as well as small amounts of various metals. The advantage of this method is that it is very simple and fast.
This method is widely used to determine the reaction of iron ions in organic reagents. Usually, for the reaction, colored compounds of cationic and anionic ions of iron in solution enter into salt-forming and complex-forming reactions.
Not all organic reagents used for the photometric determination of iron have selectivity. Therefore, it is very important to create reagents with high selectivity and analytical ability [
1].
Complex compounds of metals with hydroxy acids are of considerable interest for various fields of chemistry and chemical technology. Various hydroxy acids are able to form such complex compounds with more than 60 elements of the periodic system. At present, the study of the properties of this group of compounds is still far from complete. Nevertheless, there is already enough experimental material for some generalizations about the regularities of the complex formation process. In this article, only the complex compounds of the hydroxy acids of the fatty series are considered. The most typical and widespread representative of such hydroxy acids is tartaric acid; therefore, the main attention is paid to metal tartrate complexes. In some cases, in order to elucidate the influence of the structure of hydroxy acids on the chemical properties of complex compounds, the literature data on the complexes of citric, trihydroxyglutaric, and some other hydroxy acids are also used.
Even in the last century, it was noticed that the cations of many heavy metals in the presence of tartaric acid lost the ability to precipitate with an alkali in the form of insoluble hydroxides and changed other characteristic analytical properties. Qualitative studies have shown the connection of these phenomena with the hydroxy groups of tartaric acid.
It turned out, in addition, that other organic substances containing hydroxy groups also exhibited a similar masking effect; however, the replacement of hydroxy groups with basic or acidic residues, such as HS~, CN~, -NH2, Cl~, etc., led to a significant loss of complexing ability. Thus, the special role of hydroxy groups in the process of complex formation was elucidated.
Photometric and spectrophotometric methods, mainly the formation of colored compounds that absorb light in the visible region of the spectrum, are not very specific to fatty hydroxy acids. Photometric measurements were used to study copper, vanadium, chromium, uranium, iron, cobalt, nickel tartrate, and nitrate complexes. These works in some cases made it possible not only to determine the composition of complex compounds in solutions, but also to determine the corresponding values of instability constants [
3].
Typically, the optical density of a series of solutions with a constant concentration of the central ion and a variable concentration of the hydroxy acid is measured to determine the composition of the compound. The isomolar series method was also used. Both of these options were mainly suitable for determining the composition of complexes under conditions where the acidity of the solution was sufficient to prevent the hydrolysis of the metal salt. An auxiliary complexing agent is sometimes added to the solution to prevent hydrolysis at a high pH. As a last resort, for example, sodium salicylate solution was used in the study of tartrate and citrate complexes of iron using the method of isomolar series.
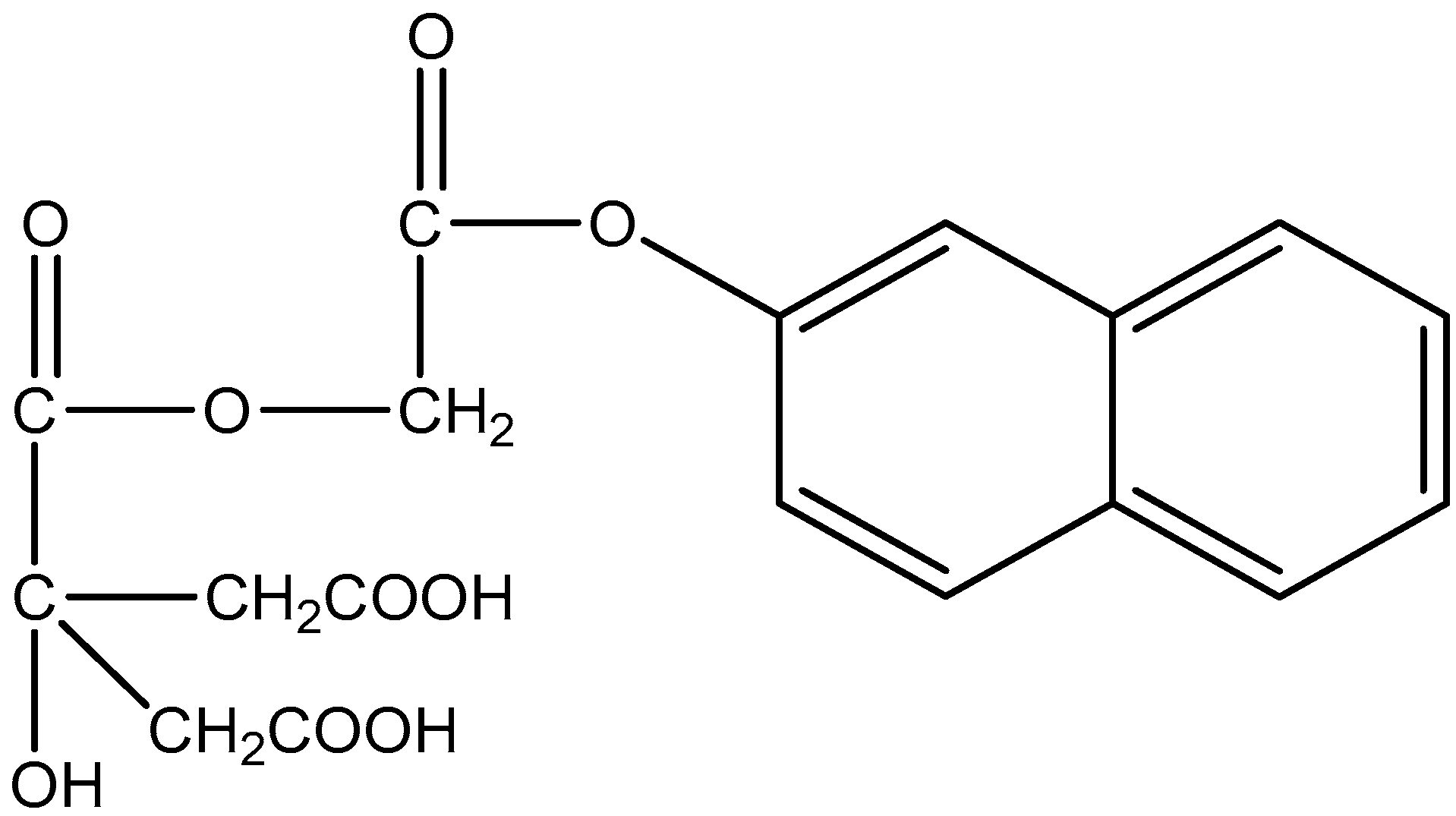
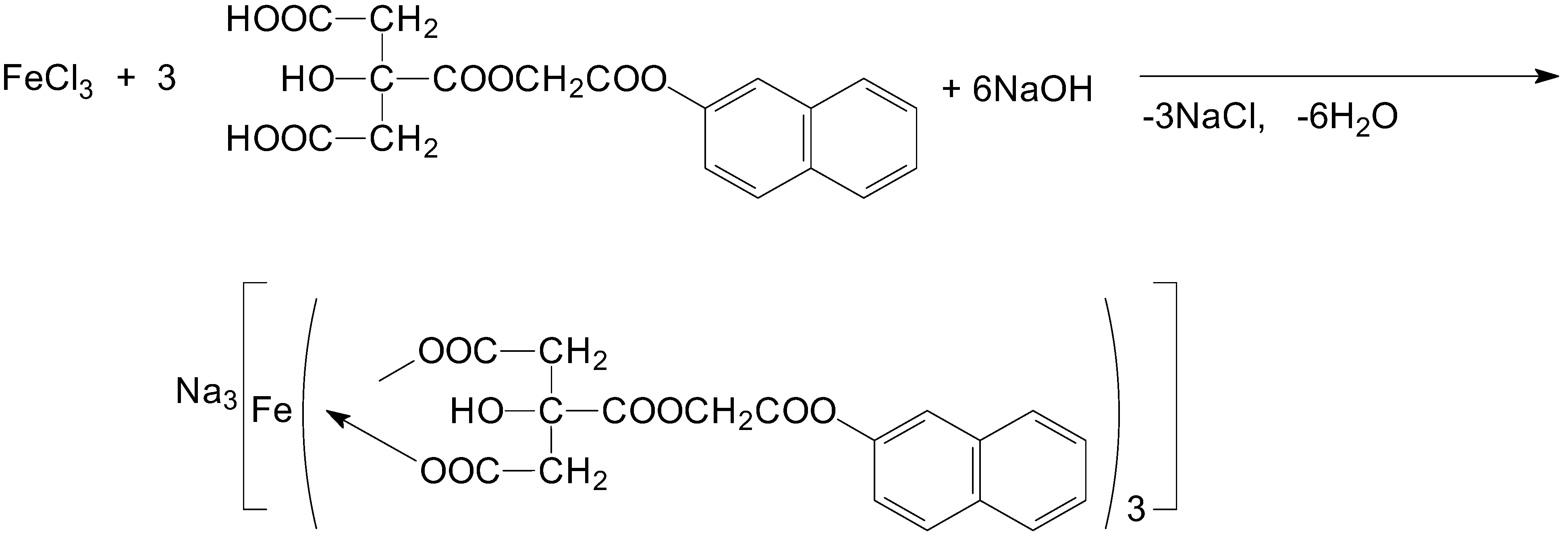
As a result of a review of the literature materials, it became known that compounds containing oxycarbonic acid and aromatic rings have high analytical ability. Taking this into account, in this work, naphthalene-2yl 2-chloroacetate was formed as a result of the reaction of naphthol-2 with chloroacetyl chloride. The resulting naphthalene-2yl 2-chloroacetate was subjected to a nucleophilic exchange reaction with the monopotassium salt of citric acid. As a result of the above processes, the reagent 2-naphthylcarboxymethylene citrate (according to systematic nomenclature it is named as follows: 3-hydroxy-3-((2-naphthalen-2-yloxy)-2-oxoethoxy) pentanedioic acid (ChemOffice12)) was formed, and the analytical capabilities of this substance were used for the purpose of the photometric determination of Fe(III) [
4,
5].
2. Experiment
2.1. Materials and Measurements
It is known that organic reagents used in analytical chemistry contain electron-donating groups (-CH
3; -C
2H
5; -OH; -OCH
3, etc.), and such reagents are very. If they contain electron-accepting groups (-SO
3; -NO
2; -COOH; CN-; CHO-, etc.), then the description of selective influence is improved. If both electron-donor (ED) and electron-acceptor (EA) groups are introduced into the analytical reagent molecule at the same time, the color of the reagent is clearly visible and the analytical and metrological characteristics of the complex are improved [
6,
7]. Such reagents increase in sensitivity and selectivity. In this scientific work, when preparing a new 0.1% solution of 2-napthylcarboxymethylene citrate sensitive (
Figure 1), 0.1 g was taken from it, put into a 100 mL measuring flask and prepared by dissolving it in distilled water up to the mark.
Initially, a standard solution of iron(III) was prepared as a working solution. For this, Fe
2(SO
4)
3∙6H
2O k.t.-branded salt was used. A total of 4.5478 g of the calculated amount of salt was weighed on an analytical balance and put into a 1000 mL measuring flask. A 1 mg/mL (1000 μg/mL) solution was prepared by dissolving it in distilled water. Working solutions were prepared by diluting an aliquot of 1 mg/mL standard solution before each work [
8,
9].
2.2. Selection of Optimal Conditions for Iron(III) Complexation with 2-Naphthylcarboxymethylene Citrate Reagent
Spectrophotometric determinations are performed under optimal conditions that ensure the complete formation of the analytical form in the solution and no or minimal deviation from the Bouguer–Lambert–Behr law. The most important of them are the optimal value of pH, the sufficient amount of the reagent, the selectivity of the analytical (photometric) reaction, and the most favorable conditions for light absorption.
In order to choose the optimal value of pH, the effect of pH on the intensity of the color of the solution at a certain wavelength was studied when the concentrations of the tested substance and reagent were unchanged. In this case, when the reagent is colorless, the area where the absorbance is the largest is considered. In colored solutions, the most favorable conditions correspond to the largest difference between the absorbances of the analytical form and the starting reagents. Under optimal conditions, small changes in pH have virtually no effect on the absorbance of the solution when the absorbance is at its maximum. Photometrically, the pH of the solution being analyzed is kept constant by using appropriate buffer solutions or sufficient amounts of acid or alkali.
The amount of analytical reagent to be added should be sufficient to convert all of the analyte within a given concentration range into analytical form. Adding more reagent does not increase the yield of the reaction product and does not increase the light absorption of the solution.
In spectrophotometric analysis, the solution must remain completely soluble in the entire range of detectable concentrations. If this condition is not met, it is necessary to use lower concentrations or to use protective substances that prevent the formation of a solid phase. Sometimes it is necessary to change the entire spectrophotometric detection scheme.
2.3. Selection of an Optimal Light Filter for Fe(III) Complexation with 2-Naphthylcarboxymethylene Citrate Reagent
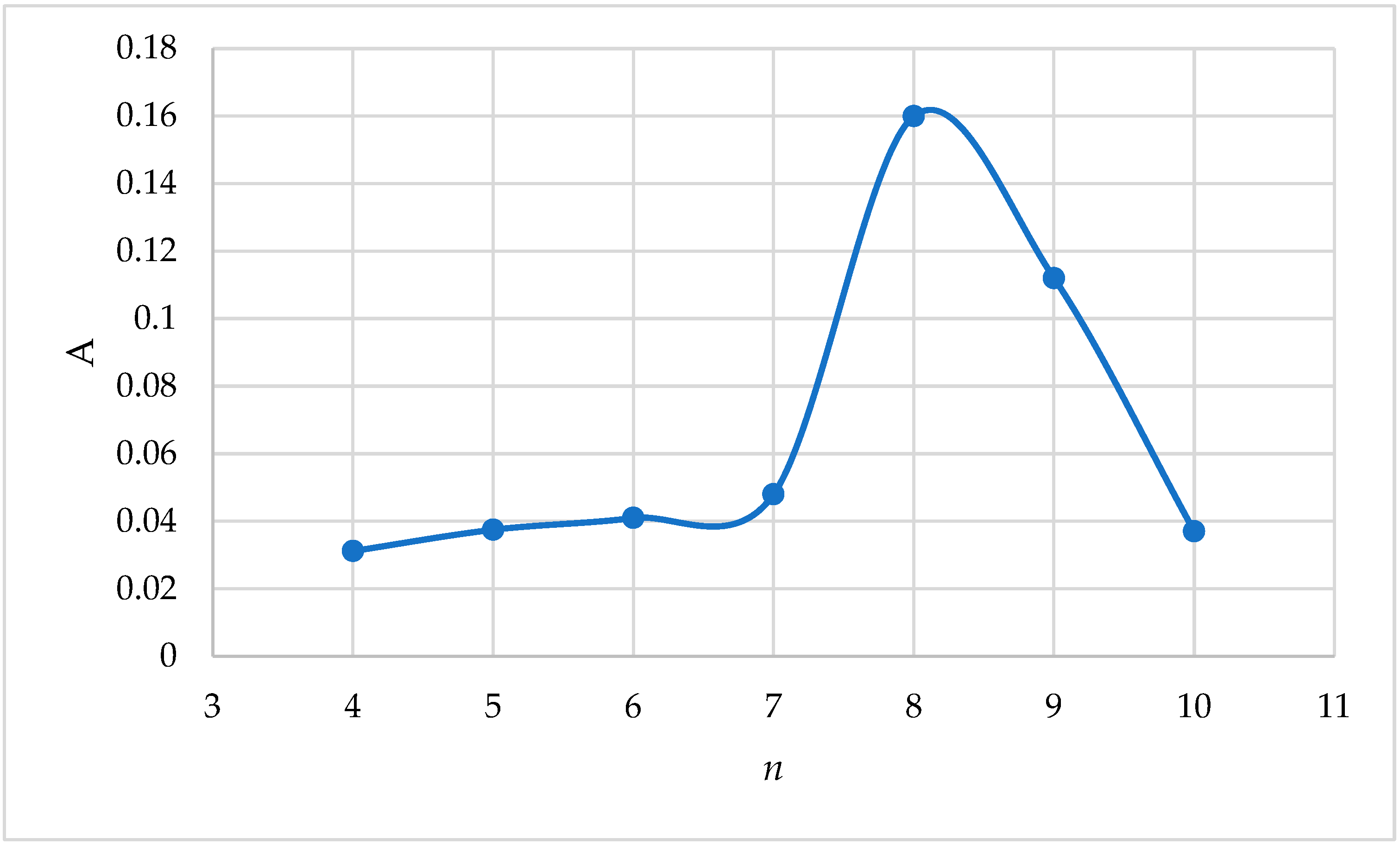
It is known that each substance by its nature absorbs light of a certain wavelength, taking into account that the highest absorption area of the complex of iron(III) with 2-naphthylcarboxymethylene citrate was determined as follows: 5.0 mL of buffer solution, 1.0 mL of 0.1% 2-naphthylcarboxymethylene citrate solution, and 1.0 mL of 20 μg/mL Fe(III) solution were added to a 25 mL volumetric flask and filled with distilled water up to the mark of the flask. The optical density of the resulting complex was measured in a concentration photoelectrocolorimeter CPhC-3 in different light filters with a light absorption thickness of
ℓ = 3.0 cm. A solution containing all components except iron(III) was used as a reference solution. The measurement results are presented in
Table 1,
Figure 2.
As can be seen from the obtained results, the complex compound 8-light filter showed high optical density at . Further work was carried out at
2.4. Dependence of the Optical Density Value of the Fe(III) Complex with 2-Naphthylcarboxymethylene Citrate Reagent on Solution Medium (pH)
Taking into account that one of the important conditions for the reaction is its environment, universal buffer solutions with different pH values were prepared for choosing optimal conditions for the complex combination of iron(III) with 2-naphthylcarboxymethylene citrate [
10,
11,
12].
To perform this, add 5.0 mL of a universal buffer solution with a pH of 2.0 to 11.10, 1.0 mL of a 0.1% 2-naphthylcarboxymethylene citrate reagent solution and 20 μg/mL of Fe(III) to a 25 mL volumetric flask. The solution was added to 1.0 mL and the flask was filled with distilled water up to the mark. The optical densities of complex compound solutions were measured in CPhC-3 cuvette at
and absorption thickness
.
Table 2 shows the obtained results.
As can be seen from
Table 2, the highest optical density of the complex compound was observed in the range of pH = 10.55–12.0, and pH = 11.80 was chosen as the optimal environment because the optical density at this pH has the maximum analytical signal. A buffer solution with a pH of 11.80 was used in further research.
2.5. Dependence of the Optical Density of the Complex Compound on the Composition of the Buffer Solution
Universal and sodium citrate buffer solutions with pH=11.80 were prepared to study the dependence of buffer solution composition on the components of the main reaction (Fe3++2-napthylcarboxymethylene citrate).
For the preparation of photometric solutions, add 5.0 mL of various buffer solutions with pH = 11.80, 1.0 mL of 0.1% aqueous solution of 2-naphthylcarboxymethylene citrate and 1.0 mL of 20 μg/mL Fe(III) solution and fill the flask up to the mark with distilled water. Optical densities were measured against the reference solution in CPhC-3,
cuvette. The obtained results are presented in
Table 3.
As can be seen from the obtained experimental results, when the universal buffer solution was used, the complex compound solution had the maximum optical density. A universal buffer solution with pH = 11.80 was used in further research.
2.6. Study of the Stability of the Fe(III) Complex with 2-Naphthylcarboxymethylene Citrate Reagent over Time
To determine the stability of the complex, the stability of the optical density of the solution with respect to time was studied.
Add 5.0 mL of universal buffer solution (pH = 11.80), 1.0 mL of 0.1% 2-naphthylcarboxymethylene citrate reagent and 1.0 mL of 20 μg/mL Fe(III) solution to a 25 mL volumetric flask. The flask was filled to the mark with distilled water. The optical density of the resulting complex was measured against a reference solution at certain time intervals. The obtained results are presented in
Table 4.
The experimental results (
Table 4) show that the optical density value of the complex compound did not change much for 2 h, and then a slight decrease was observed. It can be concluded that this time interval is sufficient to perform the analysis.
2.7. Study of the Pouring Order of the Structural Components of the Complex Compound (FeR)
Taking into account that the yield of the reaction also depends on the order of pouring the components, the solutions were prepared using the above-mentioned method, and several experiments were carried out by changing the pouring order of the components. The measurement results are presented in
Table 5.
It can be concluded from the obtained results that the pouring order of components is quite important. In further research work, the 9th casting order was chosen. Carrying out the reaction in this order ensured that the complex exhibited maximum optical density.
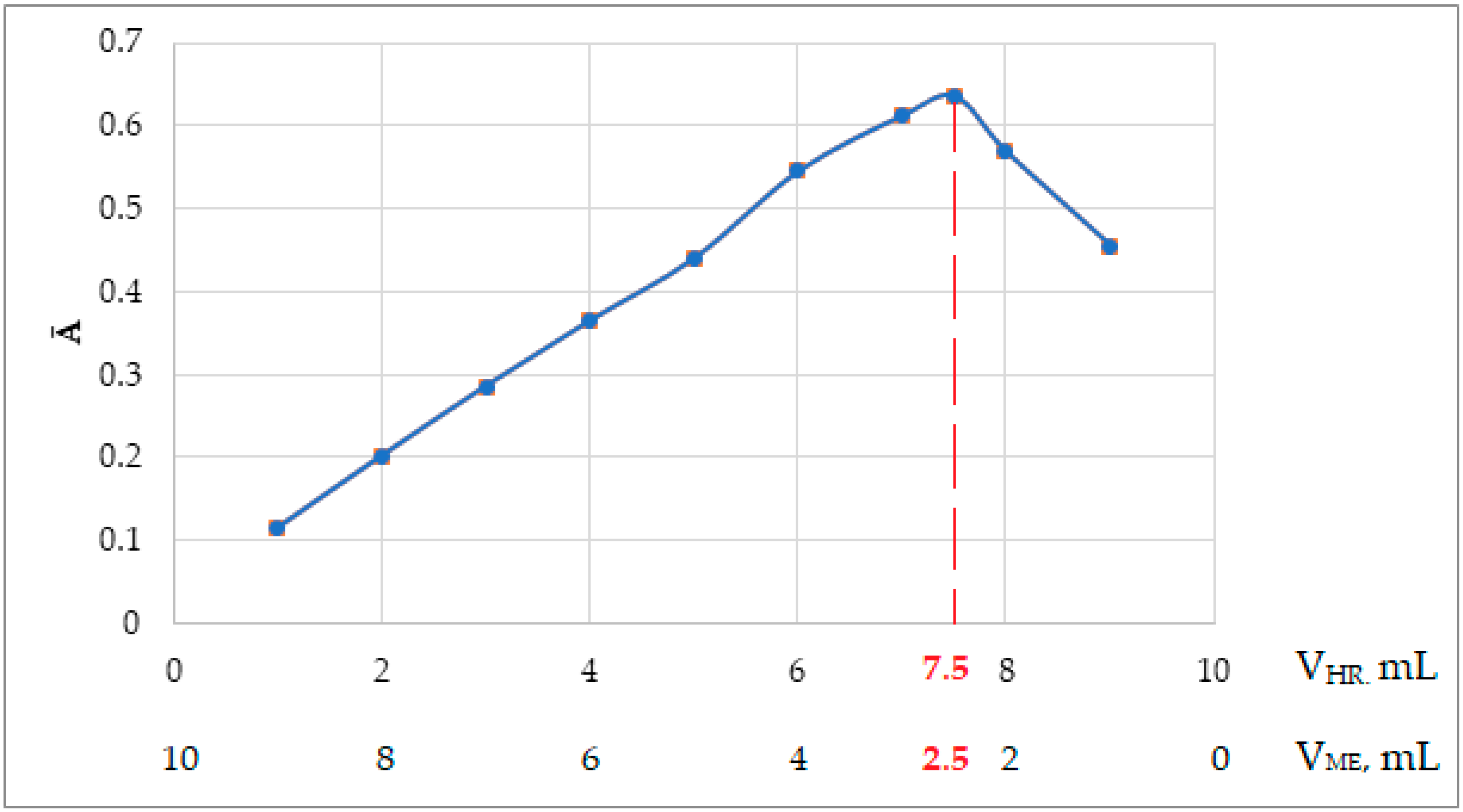
2.8. Dependence of the Optical Density of the Complex Compound on the Amount of Added Reagent
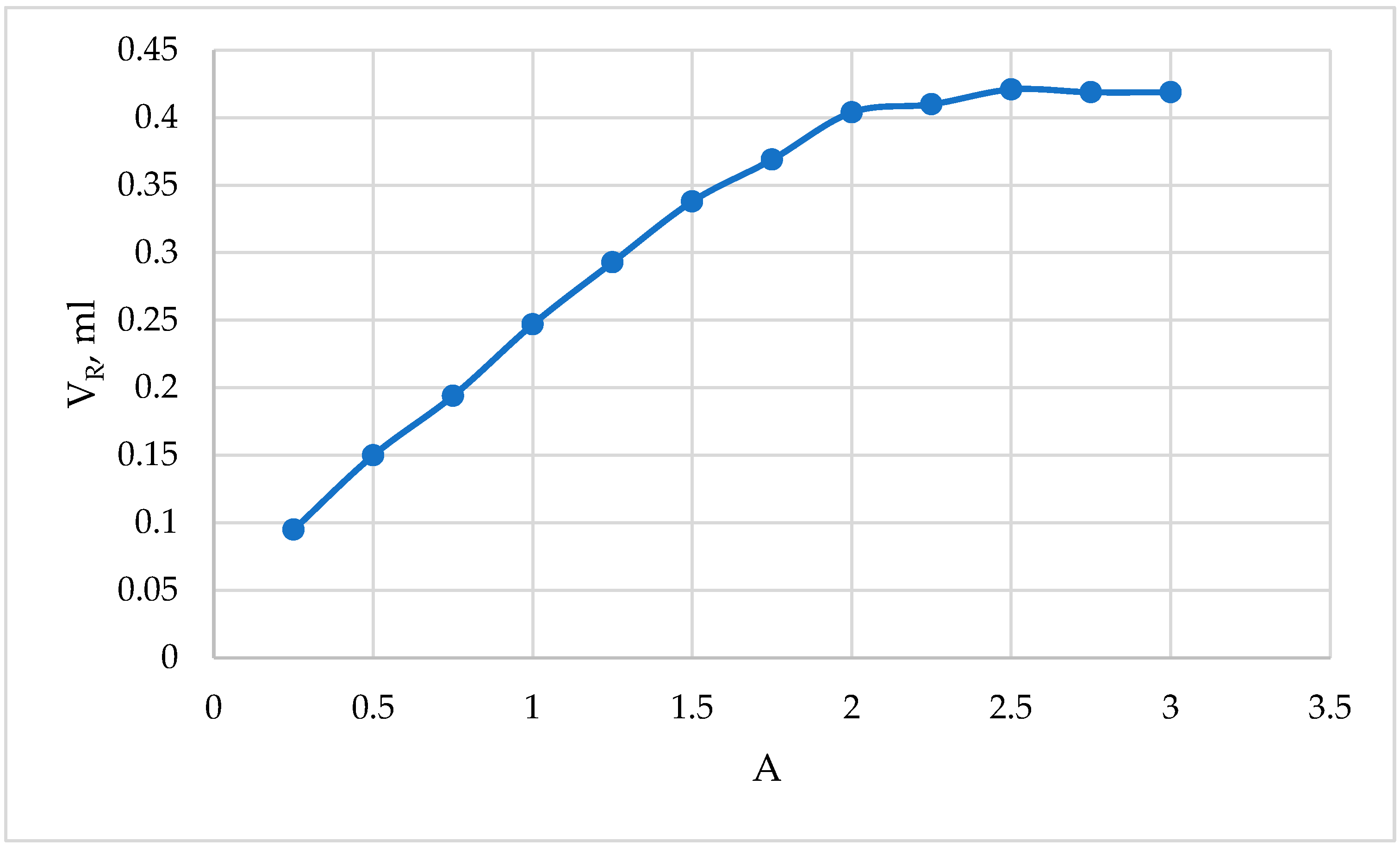
In practical studies, an excess of reagent is usually taken to fully bind the metal to the complex. For this purpose, to study the dependence of the optical density of the complex compound on the amount of added reagent, photometric solutions were prepared in 25 mL measuring flasks.
In total, 1.0 mL of a 20 µg/mL Fe(III) solution was put into 25 mL volumetric flasks with variable amounts from 0.20 to 4.00 mL of 0.1% aqueous solution of 2-naphthylcarboxymethylene citrate, 5.0 mL (pH = 11.80), and mixed with distilled water up to the mark of the flask. The optical density of the complex compound was measured in CPhC-3, Nf №4,
against the reference solution. The obtained results are presented in
Table 6 and
Figure 3.
The obtained results show that 2.5 mL of 0.1% reagent, i.e., 2.5 mL of aqueous solution of 2-napthylcarboxymethylene citrate, is sufficient for the complete binding of 20 μg/mL of iron(III) to the complex. In subsequent work, a 0.1% solution of the reagent was prepared and used in a volume of 2.5 mL.
2.9. Dependence of the Complex of Iron(III) with 2-Naphthylcarboxymethylene Citrate on the Amount of Elements (Subordination to the Bouguer–Lambert–Behr Law)
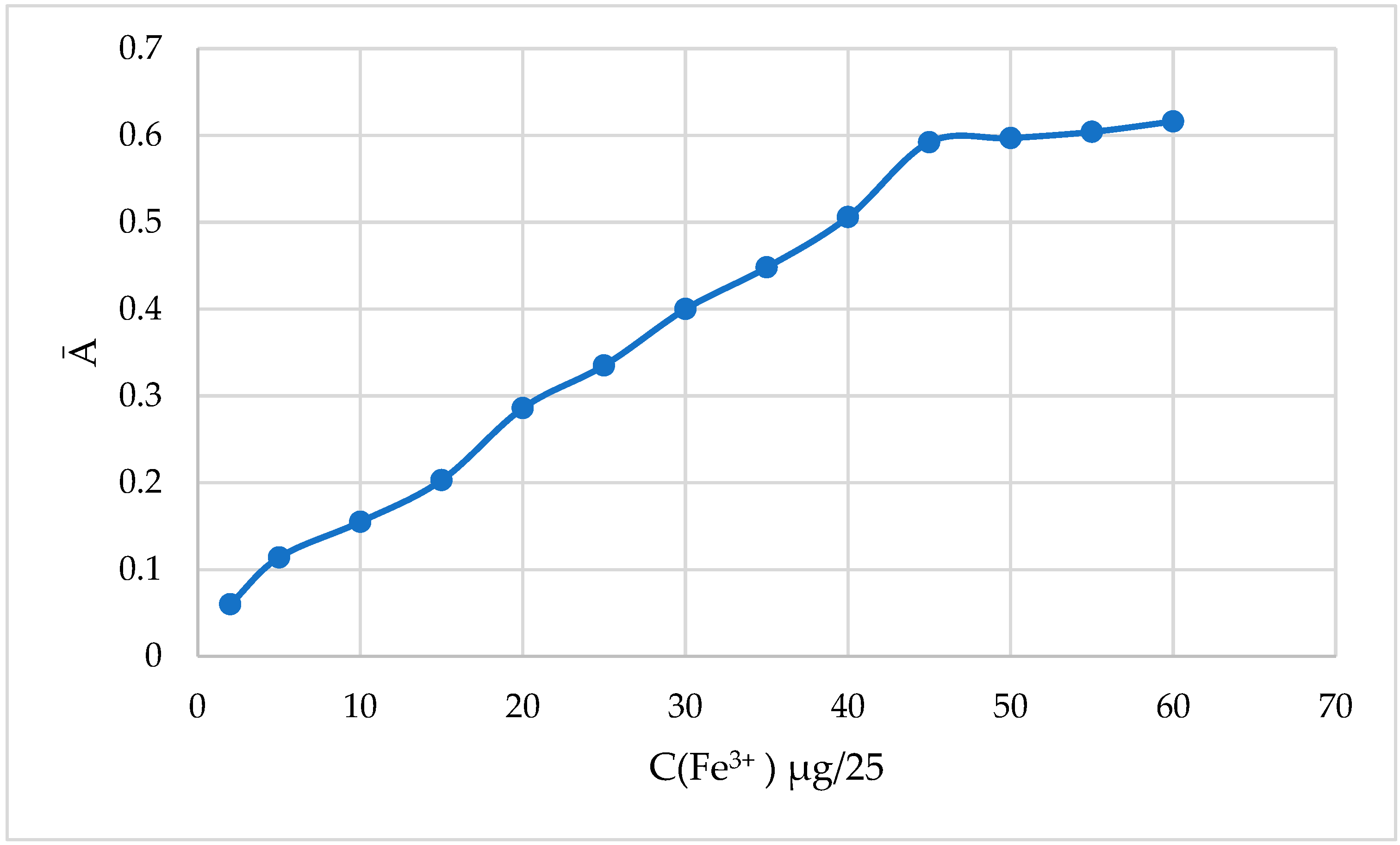
The behavior of the solution of the complex solution of iron(III) with 2-naphthylcarboxymethylene citrate was studied under selected optimal conditions.
We filled 25 mL volumetric flasks was with varying amounts of a 20 μg/mL solution of Fe(III), 2.5 mL of a 0.1% 2-naphthylcarboxymethylene citrate solution, and 5.0 mL of a universal buffer solution of rN=4.52 up to the mark of the flask and diluted it with distilled water. By mixing the solutions, their optical density (
ℓmax = 670 nm,
ℓ = 3.0 cm) was measured against the reference solution. The obtained results are presented in
Table 7 and
Figure 4.
As can be seen from the obtained results, the field of obedience to Bouguer–Lambert–Behr law was observed in the range of 2–45 μg/25 mL in 25 mL solution. At higher concentrations, there was a deviation from the linear relationship, that is, the range from 2 μg to 45 μg/25 mL allows for the quantitative determination of Fe(III).