Abstract
Deep eutectic solvents (DESs) have unique physical and chemical properties, such as low vapor pressure, ease of synthesis, stability, and non-toxicity. Although they have found application in areas of research such as organic synthesis, electrochemistry, biocatalysis, and the development of biosensors, their use as sensitive coatings for chemical sensors has not been previously considered. This study examines the fundamental principles of generating sensitive coatings for piezoelectric quartz sensors utilizing hydrophilic deep eutectic solvents (choline + polyalcohols). Thin films from DESs with a melting point above 50 °C, including those in the composite coatings with amorphous silicon oxide, have been studied. The sorption characteristics of the coatings were thoroughly examined via piezoelectric quartz microbalance. It has been demonstrated that the limits of detection and determination of volatile organic compounds in aqueous solutions by films based on DESs exhibit lower limits than other polymer coatings. A novel approach is proposed for processing the kinetic curve of the sorption of volatile substances by films based on DES to improve the reliability and detection of volatile compounds in the gas phase above aqueous solutions. The use of DES-based piezoelectric quarts sensors has been demonstrated for assessing microbiological indicators of milk.
1. Introduction
Creating and developing new sensors and devices for analyzing various objects is a constantly developing area of modern sensor science. Gas sensors have a sensitive surface layer, which determines their analytical characteristics. The continuous pursuit and synthesis of novel materials to produce sensitive sensor coatings is ongoing [1,2]. Polymer sorbents [3,4], metal–organic structures [5], metal oxides and salts [6,7], carbon nanomaterials [8,9], composite materials [10], and various combinations of the studied sorbents [11].
Deep eutectic solvents (DESs) are widely used in analytical practice for the analysis of real objects with complex composition due to their unique properties, such as low vapor pressure, ease of synthesis, stability, non-toxicity, and the ability to control the degree of hydrophilicity by choosing initial components. Therefore, their use as sorbents for creating coatings for gas sensors is promising for extracting volatile components of different polarity from the gas phase.
The goal is to investigate the sorption properties of thin films based on hydrophilic deep eutectic solvents (choline + polyalcohols) and their application to the analysis of the gas phase of milk.
2. Materials and Methods
2.1. Preparation of Deep Eutectic Solvents
Hydrophilic eutectic solvents based on choline bitartrate (IROOX, Kachkanar, Russia) and polyalcohols (erythritol, xylitol, and sorbitol) (Sigma–Aldrich, Burlington, MA, USA) were chosen to create sorption coatings. The thermal method was used to obtain DESs. Choline bitartrate and one of the polyalcohols were placed in a glass vessel and stirred for two hours while heating in a water bath to 80 °C. A viscous flowing transparent liquid was formed, then cooled to room temperature. The obtained deep eutectic solvents had a melting point above ambient temperature, causing the DESs to solidify to form a white plastic mass under normal conditions.
2.2. Formation and Analysis of Sensitive Layers of Sensors Based on a Deep Eutectic Solvent
To fabricate sensor coatings, a mixture of 2 mL of ethanol (95% vol.) and 10 mg of a DES was incorporated until a transparent solution was obtained. This solution was then applied to the electrode of a piezoelectric quartz resonator (16 MHz, Meteor Plant JSC, Volzhsky, Russia) via dispersion spraying [12]. Subsequently, the coating was placed in an oven at 50 °C for 20 min to remove any unbound ethanol. Moreover, to fabricate a more stable coating with a higher specific surface, 10 mg of amorphous silicon oxide (ASO) (a polydisperse powder with a particle size of up to 0.09 mm and 99% purity) was added to the resulting solution and sonicated (90 W, 60 s) to obtain a stable sol. After this, the obtained sol was also deposited on the electrode surface via dispersion spraying. The surface of the obtained coatings was examined using the Solver-Pro NT-MDT scanning tunneling microscopy.
2.3. Estimation of Volatile Compounds Sorption Features via Coating Based on a Deep Eutectic Solvent
The study of the volatile compound sorption was carried out on the device “MAG-8” (OOO “Sensors—New Technologies”, Voronezh, Russia) with piezoelectric quartz sensors and an injector input of the gas phase [13]. The sensors were trained on volatile organic compounds of various classes, including alcohols, ketones, ethyl acetate, acetaldehyde, carboxylic acids (analytical grade, Reakhim LLC, Moscow, Russia), and bidistilled water, to evaluate their sorption characteristics. The measurement time of sorption of the equilibrium gas phase over pure compounds or samples of raw milk (20 mL) was 80 s. A software of MAG-8 recorded the values of the shift of frequency of the piezoelectric sensor during the sorption of the volatile compounds with a frequency of 1 s, according to which the maximum sensor signal (ΔFmax,i, Hz) was obtained. The effectiveness of sorption via the obtained coatings was evaluated using specific mass sensitivity [11] and a selectivity coefficient [12].
Based on the sensor signals after the measurement, the parameter β has been proposed, which was calculated using the equation:
where Fmax,i and ΔF80s,i are the maximum signal and the signal at 80 s of measurement for the i-th sensor, respectively, and τmax,i—time of achievement of the maximum signal by the i-th sensor. The proposed parameter reflects the sorption kinetics of volatile compounds on piezosensor coatings and can be used to identify the volatile compounds in the gas phase over samples. In order to determine the detection limit of volatile compounds in aqueous solutions, we studied the gas phase sorption of certain substances (acetic and butyric acids, butanone-2, and isopentanol) in the concentration range of 0.001–10% by volume.
βi = (ΔFmax,i − ΔF80s,i)/(80 − τmax,i),
2.4. Milk Analysis
Samples of raw cow’s milk (n = 14) were obtained from various farms at different seasons (March–July 2023), cooled immediately after milking to T = (4 ± 2) °С, and delivered to the laboratory for no more than 3 h of storage.
2.4.1. Determination of Physical and Chemical Properties of the Milk
Mass fraction of dry solids in the samples was determined by drying [14] in a Binder ED 53 oven (BINDER Inc., Tuttlingen, Germany) to a constant mass at T = (105 ± 2) °С; the mass fraction of fat—using the Gerber acid method [15], mass fraction of total protein—using formol titration [16], density—using the areometric method [17], titratable acidity—using the titrimetric method with phenolphthalein indicator [18], and purity group—using the gravimetric method [19]. The experimental studies of each sample were carried out three to five times. The number of repetitions of each experiment to determine one value was three times.
2.4.2. Determination of Microbiological Indicators
Microbiological indicators (the quantity of mesophilic aerobic and facultative anaerobic microorganisms QMAFAnM and the quantity of yeasts and molds) were determined using microbiological inoculation on universal nutrient media (plate count agar, Sabouraud agar, Obolensk, Russia) according to standard methods described in GOST [20,21]. QMAFAnM was estimated as the average value from three milk dilutions (from 106 to 104). The raw milk sample was diluted 10 times to estimate the quantity of yeast and mold.
2.5. Data Processing
Calculations were carried out using methods of mathematical statistics via the XLSTAT application (Lumivero, Denver, CO, USA) for Microsoft Office 365 Family (Microsoft Corporation, Redmond, WA, USA). The significance of the findings was determined by utilizing the p-value, which was less than or equal to 0.05. The detection limit of volatile compounds is estimated by the difference (three criteria) between the sensor signal for sensor vapor and the sensor signal for water vapor. To assess the correlation of the output data of the sensors with physical, chemical, and microbiological indicators, the Pearson correlation coefficient was calculated with an assessment of its statistical significance via the Student’s t-criteria [22].
3. Results
When analyzing the gas phase over aqueous solutions, the resulting coatings based on deep eutectic solvents show different levels of baseline drift during three months of intensive operation (Table 1).

Table 1.
Performance characteristics of sensor coatings and specific mass sensitivity of sensor coatings to vapors of some test substances.
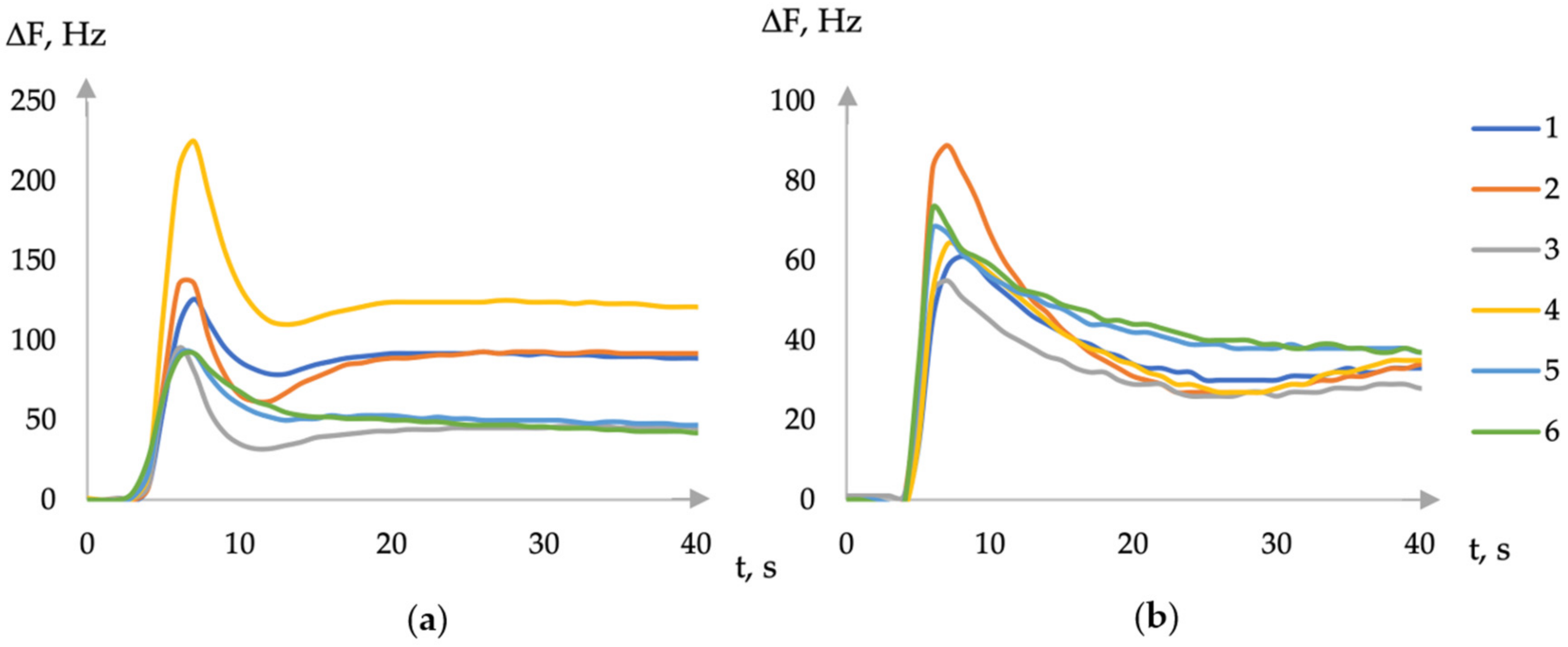
According to the type of chronofrequencyograms, different nature of the sorption of volatile compounds on DES films was established, depending on the ability of the substance to form hydrogen bonds, for example, the sorption of ethanol and acetone vapors (Figure 1).
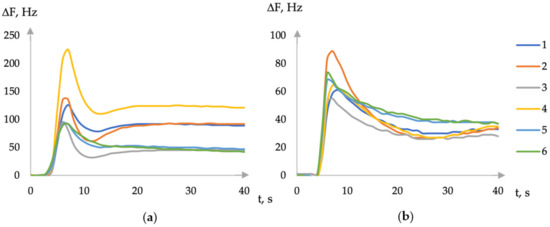
Figure 1.
The first part of chronofrequencygram of ethanol (a) and acetone (b) vapor sorption on an array of sensors with coatings (1—choline + xylitol, 2—choline + erythritol, 3—ASO + choline + erythritol, 4—choline + sorbitol, 5—ASO + choline + sorbitol, and 6—ASO + choline + xylitol).
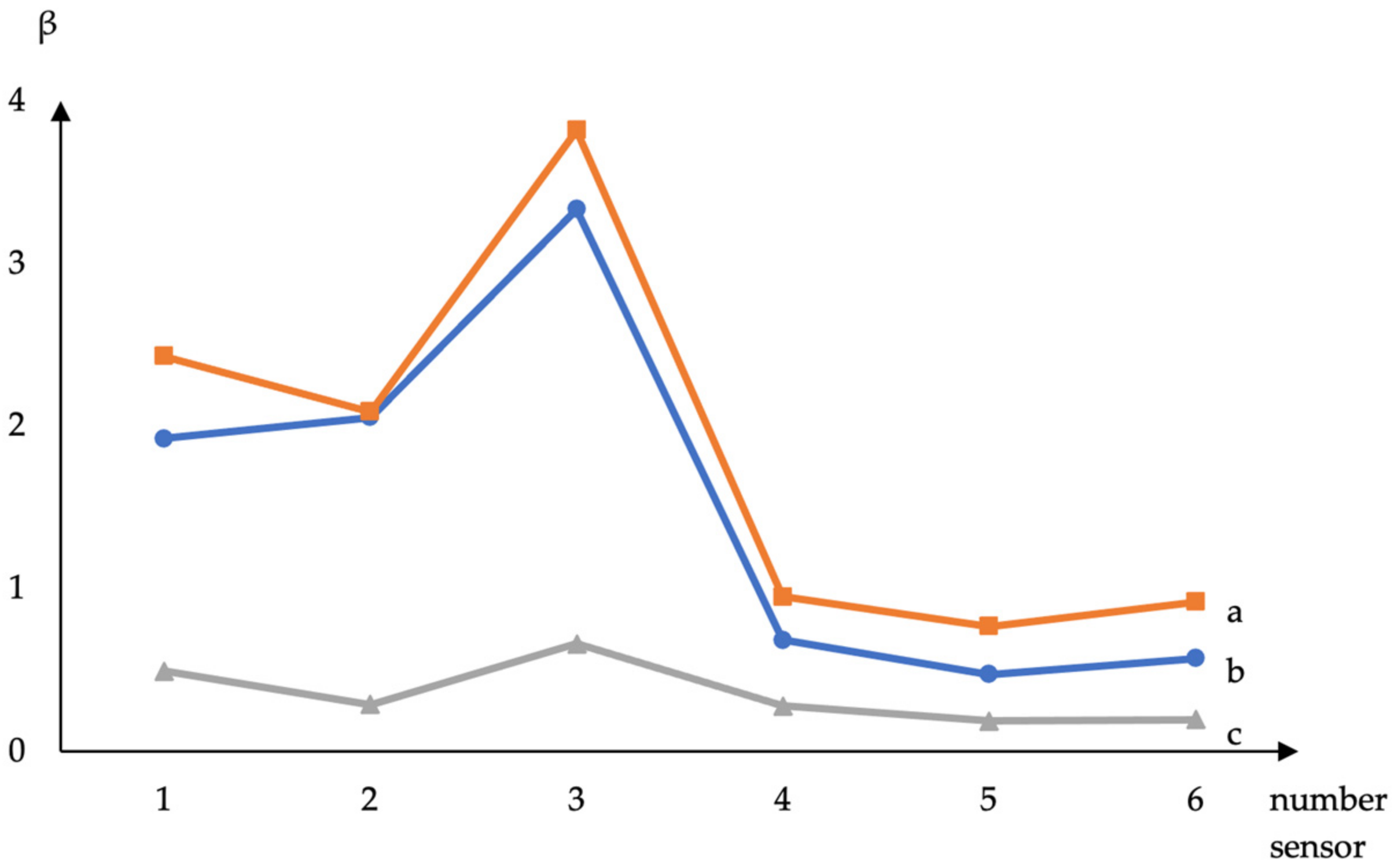
The parameters β for volatile substances were calculated from the signals of sensors with films based on DESs (Figure 2).
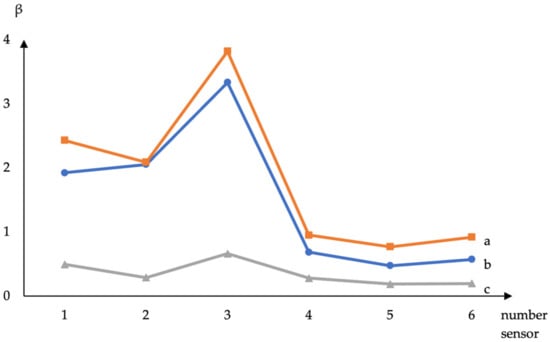
Figure 2.
Calculated from sensor signals (1—choline + xylitol, 2—choline + erythritol, 3—ASO + choline + erythritol, 4—choline + sorbitol, 5—ASO + choline + sorbitol, and 6—ASO + choline + xylitol) parameter β for volatile compounds: a—ethyl acetate, b—acetic acid, and c—methyl ethyl ketone.
The statistically significant correlations (r) between signals and sensor parameters and the outcomes of determining the microbiological parameters of milk samples were established (Table 2).

Table 2.
Statistically significant correlation coefficients (r) of sensor signals and microbiological parameters of milk samples.
The results of determining the physical, chemical, and microbiological parameters of milk are presented in Table A1 (Appendix A).
4. Discussion
It has been established that adding amorphous silicon oxide increases the stability of the film on the electrodes of the piezoresonator, therefore increasing the service life of sensors based on them. Additionally, the sorption efficiency of volatile compounds is increased (Table 1). It has been demonstrated that the reduction of the specific mass sensitivity of the micro-weighing of vapors of volatile compounds is associated with the increase in the carbohydrate radical of polyalcohol in the composition of the DES (Table 1). Furthermore, the addition of silicon oxide abnormally increases the mass-specific sensitivity of micro-weighing with a choline + xylitol + ASO film. This phenomenon is believed to be attributable to the more effective distribution of DESs on silicon oxide particles during the coating formation process and the steric availability of hydroxyl groups of xylitol for sorption. Despite the lower mass, the sorption efficiency on the choline + xylitol + ASO film is higher for polar volatile compounds with a hydrocarbon radical length exceeding C3 (Table 1).
The addition of amorphous silicon oxide in the formation of a film with DES partially eliminates the differences in the chronofrequencygram of proton and aprotic substances (Figure 1). The length of the hydrocarbon radical of polyalcohol, which is part of the DES, does not affect the type of chronofrequency gram. Therefore, the kinetic features of sorption are also retained in this case. The study of the absorption of volatile vapors in the gas phase over aqueous solutions revealed that, for all films based on DES, the sensitivity of micro-weighing of vapors of substances abruptly changes when the concentration of the substance in the solution reaches 0.1 or 1% by volume. Based on the findings obtained from a comparative analysis of the sorption of volatile compounds in aqueous solutions for films based on DES and macromolecular sorbents (crown ethers, Triton X-100), it was determined that the detection limit of volatile compounds is significantly lower (0.001–0.01% vol.) than that of previously employed sorbents (0.01–0.1% vol.)
It has been established that the β parameter differs for substances depending on the composition of the DES. However, when ASO is added, the β parameter practically does not differ for coatings of different compositions and is close to the β parameter for an amorphous silicon oxide film. It was found that the sensor data with choline + sorbitol film correlated with the indicators of titratable acidity and the content of fungi and mold in raw milk (Table 2). The parameters of the sensor with choline + erythritol + ASO film significantly correlate with the yeast content in milk (Table 2). The addition of signals and sensor parameters into the regression model to predict the total microbial count of milk samples can increase the accuracy and reduce prediction error to 6%.
Author Contributions
Conceptualization, A.S.; methodology, A.S., E.A. and E.B.; validation, R.U., E.A. and E.B.; formal analysis, A.S. and E.B.; investigation, all authors.; writing—original draft preparation, A.S., R.U. and E.B.; writing—review and editing, A.S. and E.A.; visualization, A.S. and R.U.; project administration, R.U.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation grant no. 22-76-10048.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Korneeva O.S. for providing a microbiology laboratory for investigation and Kuchmenko T.A. for providing the equipment for gas phase analysis.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Physical, chemical, and microbiological indicators of raw milk samples.
Table A1.
Physical, chemical, and microbiological indicators of raw milk samples.
| No | Mass Fraction of Dry Solids, % | Mass Fraction of Fat, %, | Mass Fraction of Total Protein, % | Titratable Acidity, °Т | QMAFAnM *, CFU/mL | Quantity of Yeast CFU/mL | Quantity of Mold CFU/mL |
|---|---|---|---|---|---|---|---|
| 1 | 16.02 ± 0.12 | 7.5 ± 0.3 | 3.46 ± 0.15 | 19 ± 0.5 | 10,000,000 | 100,000 | 0 |
| 2 | 12.22 ± 0.13 | 3.8 ± 0.1 | 3.74 ± 0.10 | 20 ± 0.5 | 4,000,000 | 10,000 | 0 |
| 3 | 13.36 ± 0.08 | 4.8 ± 0.1 | 3.45 ± 0.10 | 19 ± 0.5 | 4,500,000 | 1000 | 10 |
| 4 | 15.15 ± 0.14 | 7.5 ± 0.5 | 3.26 ± 0.10 | 15 ± 0.5 | 340,000 | 0 | 0 |
| 5 | 11.63 ± 0.13 | 3.5 ± 0.1 | 3.01 ± 0.10 | 19 ± 0.5 | 2,400,000 | 1500 | 160 |
| 6 | 11.77 ± 0.11 | 3.1 ± 0.1 | 3.30 ± 0.15 | 19 ± 0.5 | 590,000 | 650 | 900 |
| 7 | 10.83 ± 0.09 | 3.9 ± 0.1 | 2.40 ± 0.10 | 15 ± 0.5 | 4,640,000 | 5680 | 0 |
| 8 | 12.31 ± 0.12 | 3.7 ± 0.1 | 3.10 ± 0.15 | 18 ± 0.5 | 98,000,000 | 8004 | 60 |
| 9 | 11.41 ± 0.06 | 3.2 ± 0.1 | 2.00 ± 0.05 | 15 ± 0.5 | 480,000 | 0 | 10 |
| 10 | 12.14 ± 0.10 | 4.1 ± 0.1 | 2.88 ± 0.10 | 16 ± 0.5 | 5,700,000 | 34,200 | 300 |
| 11 | 11.72 ± 0.07 | 3.4 ± 0.1 | 1.16 ± 0.10 | 15 ± 0.5 | 42,000,000 | 1800 | 0 |
| 12 | 10.92 ± 0.09 | 3.3 ± 0.1 | 1.35 ± 0.10 | 11 ± 0.5 | 2,000,000 | 2300 | 10 |
| 13 | 11.44 ± 0.11 | 3.6 ± 0.1 | 2.59 ± 0.15 | 17 ± 0.5 | 3,400,000 | 17,400 | 10 |
| 14 | 15.07 ± 0.15 | 6.5 ± 0.3 | 3.07 ± 0.10 | 16 ± 0.5 | 39,000,000 | 100,000 | 0 |
*—the number of CFU is calculated as the arithmetic mean value when counting on Petri dishes with different dilutions if it was possible or from appropriate dilution.
References
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced Micro- and Nano-Gas Sensor Technology: A Review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sikarwar, S.; Verma, A.; Yadav, B.C. The recent development of metal oxide heterostructures based gas sensor, their future opportunities and challenges: A review. Sens. Actuators A Phys. 2021, 332, 113127. [Google Scholar] [CrossRef]
- Kuchmenko, T.A.; Shuba, A.A.; Menzhulina, D.A.; Volkova, A.A.; Vecherkin, V.A.; Cornejo Tueros, J.V. On a Correlation between the Results of In-Hospital Analysis of Biosamples from Children Performed Using Standard Methods and an Array of Piezosensors. J. Anal. Chem. 2022, 77, 376–387. [Google Scholar] [CrossRef]
- Matindoust, S.; Farzi, G.; Nejad, M.B.; Shahrokhabadi, M.H. Polymer-based gas sensors to detect meat spoilage: A review. React. Funct. Polym. 2021, 165, 104962. [Google Scholar] [CrossRef]
- Yuan, H.; Li, N.; Fan, W.; Cai, H.; Zhao, D. Metal–organic Framework Based Gas Sensors. Adv. Sci. 2021, 9, 2104374. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y. Recent advances in SnO2 nanostructure based gas sensors. Sens. Actuators B Chem. 2022, 364, 131876. [Google Scholar] [CrossRef]
- Kuchmenko, T.A.; Menzhulina, D.A.; Murakhovskii, I.A. Using Highly Sensitive Piezo Sensors in an Open System for the Diagnostic Analysis of Skin Volatile Substances. J. Anal. Chem. 2023, 78, 1013–1028. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef] [PubMed]
- Fauzi, F.; Rianjanu, A.; Santoso, I.; Triyana, K. Gas and humidity sensing with quartz crystal microbalance (QCM) coated with graphene-based materials—A mini review. Sens. Actuators A Phys. 2021, 330, 112837. [Google Scholar] [CrossRef]
- Shakeel, A.; Rizwan, K.; Farooq, U.; Iqbal, S.; Altaf, A.A. Advanced polymeric/inorganic nanohybrids: An integrated platform for gas sensing applications. Chemosphere 2022, 294, 133772. [Google Scholar] [CrossRef] [PubMed]
- Shuba, A.; Kuchmenko, T.; Umarkhanov, R. Piezoelectric Gas Sensors with Polycomposite Coatings in Biomedical Application. Sensors 2022, 22, 8529. [Google Scholar] [CrossRef] [PubMed]
- Shuba, A.; Kuchmenko, T.; Umarkhanov, R.; Bogdanova, E. Composite Coatings of Piezoelectric Quartz Sensors Based on Viscous Sorbents and Casein Micelles. In Proceedings of the XVII International Research Conference Proceedings, Istanbul, Türkiye, 24–25 April 2023. [Google Scholar]
- Kuchmenko, T.; Menzhulina, D.; Shuba, A. Noninvasive Detection of Bacterial Infection in Children Using Piezoelectric E-Nose. Sensors 2022, 22, 8496. [Google Scholar] [CrossRef] [PubMed]
- GOST R 54668-2011; Milk and Milk Products. Methods for Determination of Moisture and Dry Substance mass Fraction. Standartinform: Moscow, Russia. Available online: https://internet-law.ru/gosts/gost/52063/ (accessed on 7 August 2023). (In Russian)
- GOST 5867-90; Milk and Dairy Products. Methods of Determination of Fat. Standartinform: Moscow, Russia. Available online: https://internet-law.ru/gosts/gost/2476/ (accessed on 7 August 2023). (In Russian)
- GOST 25179-2014; Milk and Milk Products. Method for Determination of Protein. Standartinform: Moscow, Russia. Available online: https://internet-law.ru/gosts/gost/58007/ (accessed on 7 August 2023). (In Russian)
- GOST R 54758-2011; Milk and Milk Products. Methods for Determination of Density. Standartinform: Moscow, Russia. Available online: https://internet-law.ru/gosts/gost/52080/ (accessed on 7 August 2023). (In Russian)
- GOST R 54669-2011; Milk and Milk Products. Methods for Determination of Acidity. Standartinform: Moscow, Russia. Available online: https://internet-law.ru/gosts/gost/52065/ (accessed on 7 August 2023). (In Russian)
- GOST 8218-89; Milk Method of Purity Determination. Standartinform: Moscow, Russia. Available online: https://internet-law.ru/gosts/gost/38652/ (accessed on 7 August 2023). (In Russian)
- GOST 32901-2014; Milk and Milk Products. Methods of Microbiological Analysis. Standartinform: Moscow, Russia. Available online: https://docs.cntd.ru/document/1200115745 (accessed on 7 August 2023). (In Russian)
- GOST 33566–2015; Milk and Dairy Products. Determination of Yeasts and Molds. Standartinform: Moscow, Russia. Available online: https://internet-law.ru/gosts/gost/61246/ (accessed on 7 August 2023). (In Russian)
- Doerffel, K. Statistics in Analytical Chemistry, 5th ed.; Dt. Verlag für Grundstoffindustrie, Cop.: Leipzig, Germany, 1990; 256p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).