A Novel Approach to Fabricating a Screen-Printed Electrode Based on a Gold Nanorod–Graphene Oxide Composite for the Detection of Uric Acid †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Graphene Oxide (GO) and Gold Nanorod (AuNR)
2.2. Formulation of Conductive Ink for SPE Fabrication
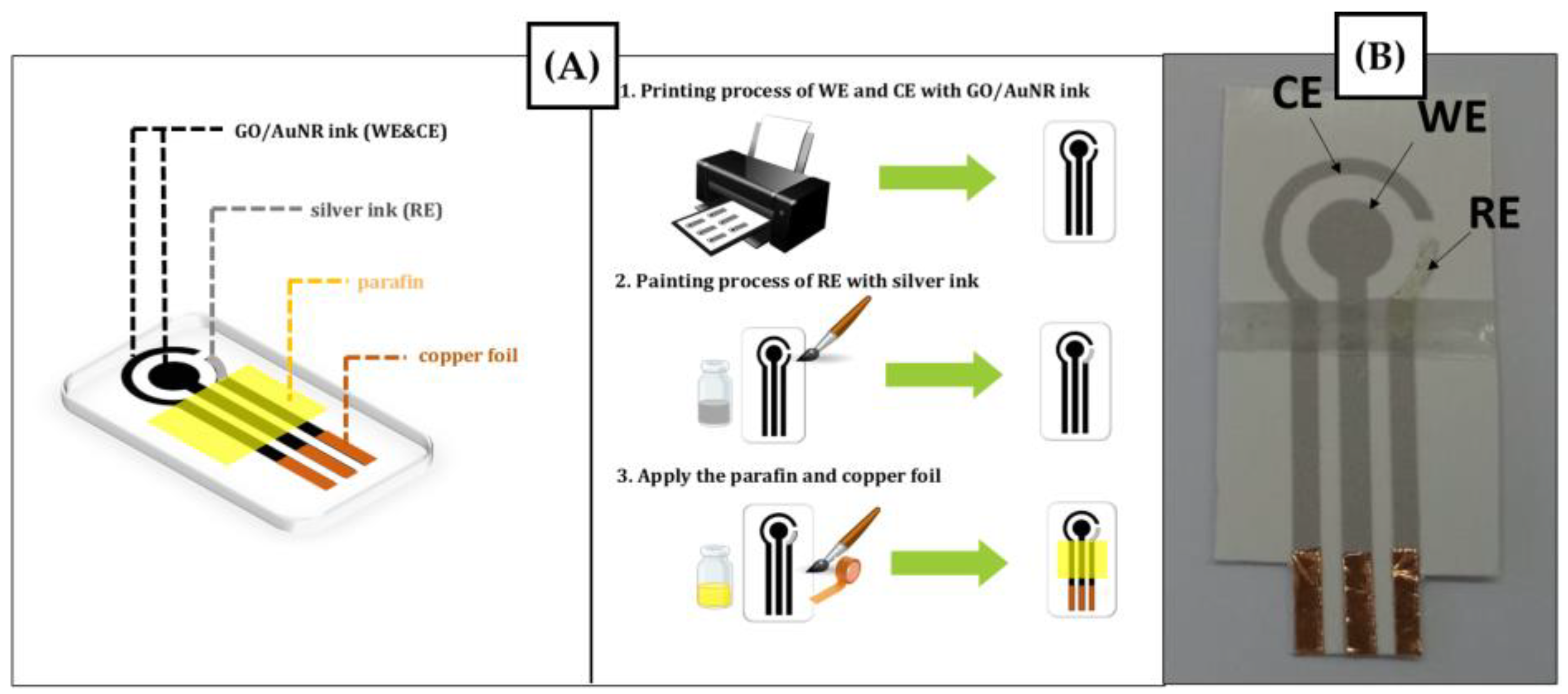
2.3. Fabrication of SPE Based on the Composite of GO/AuNR
2.4. Evaluation of the Analytical Performance of the Fabricated SPE
3. Results and Discussions
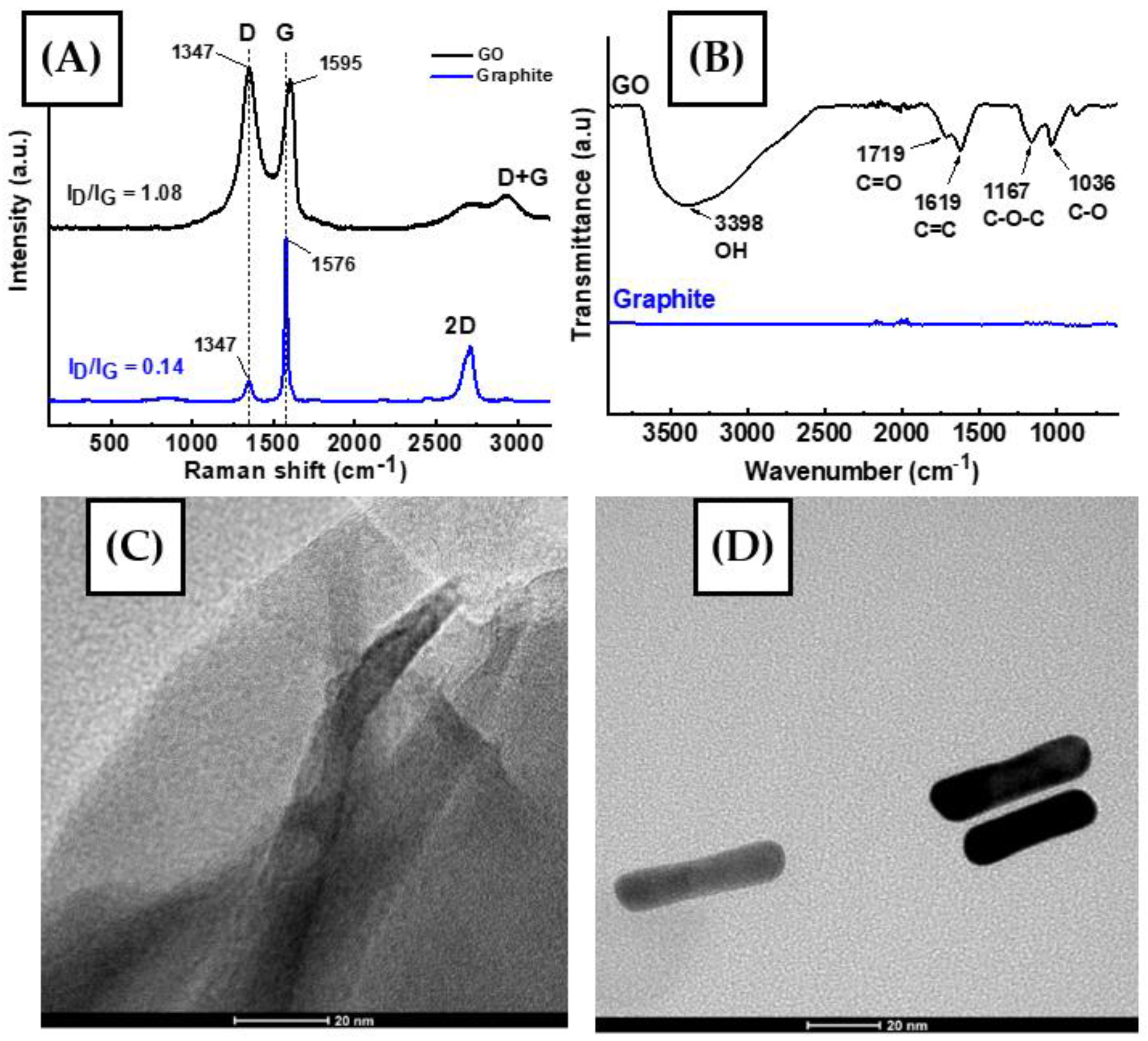
3.1. Characterization of GO and AuNR
3.2. Fabrication of SPE Based on GO/AuNR Using an Inkjet Printer
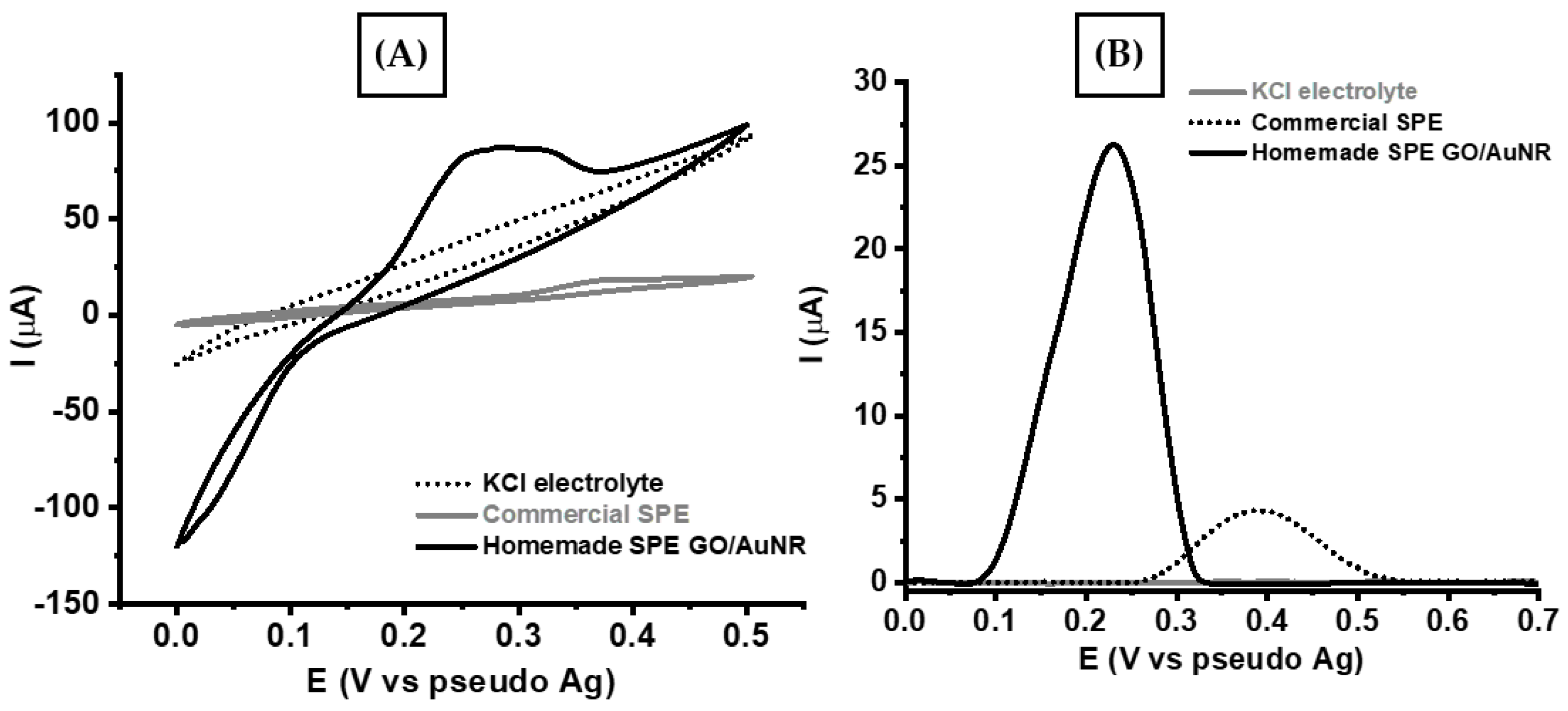
3.3. Electrochemical Investigation of Uric Acid Oxidation Using SPE-Based GO/AuNR
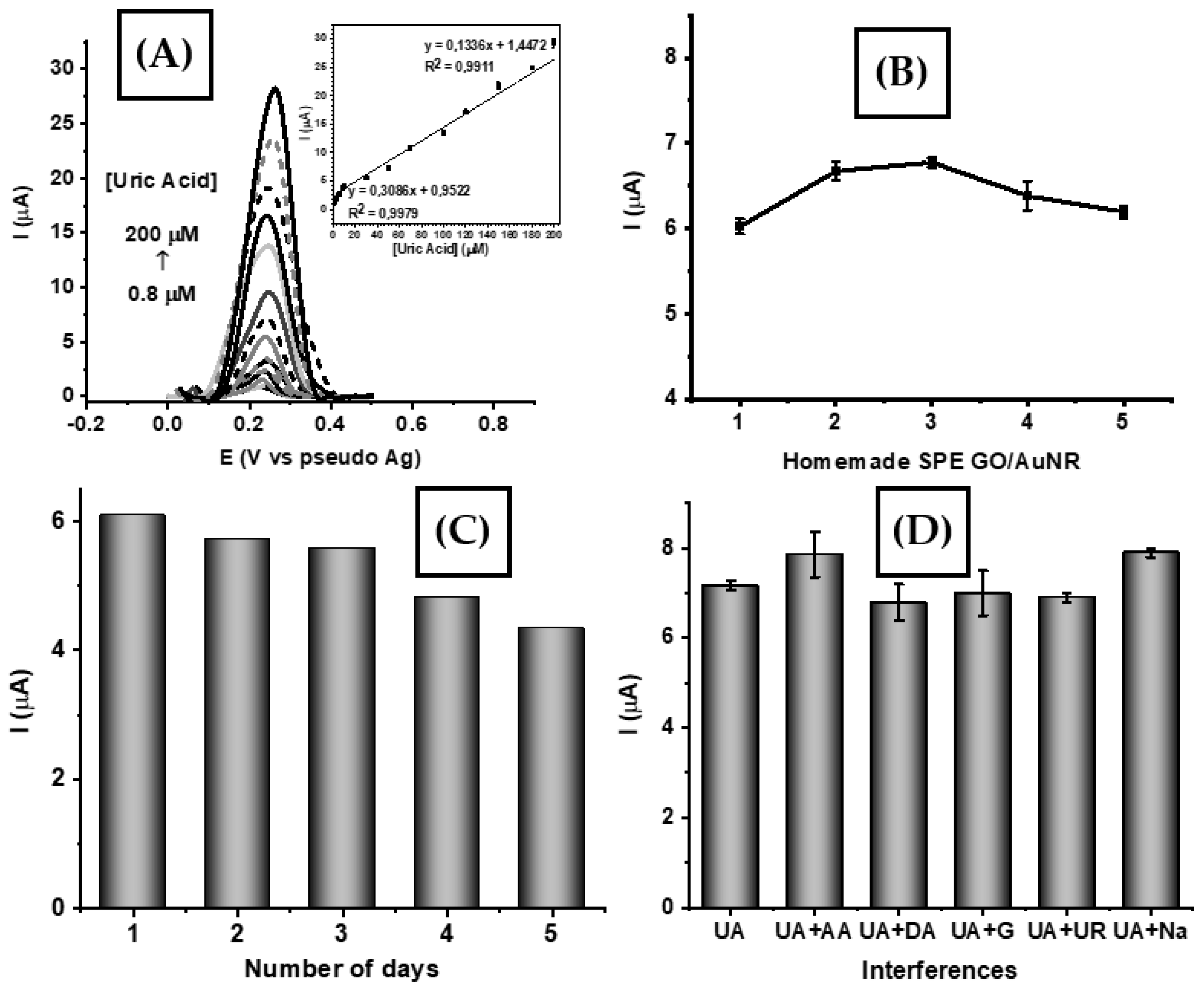
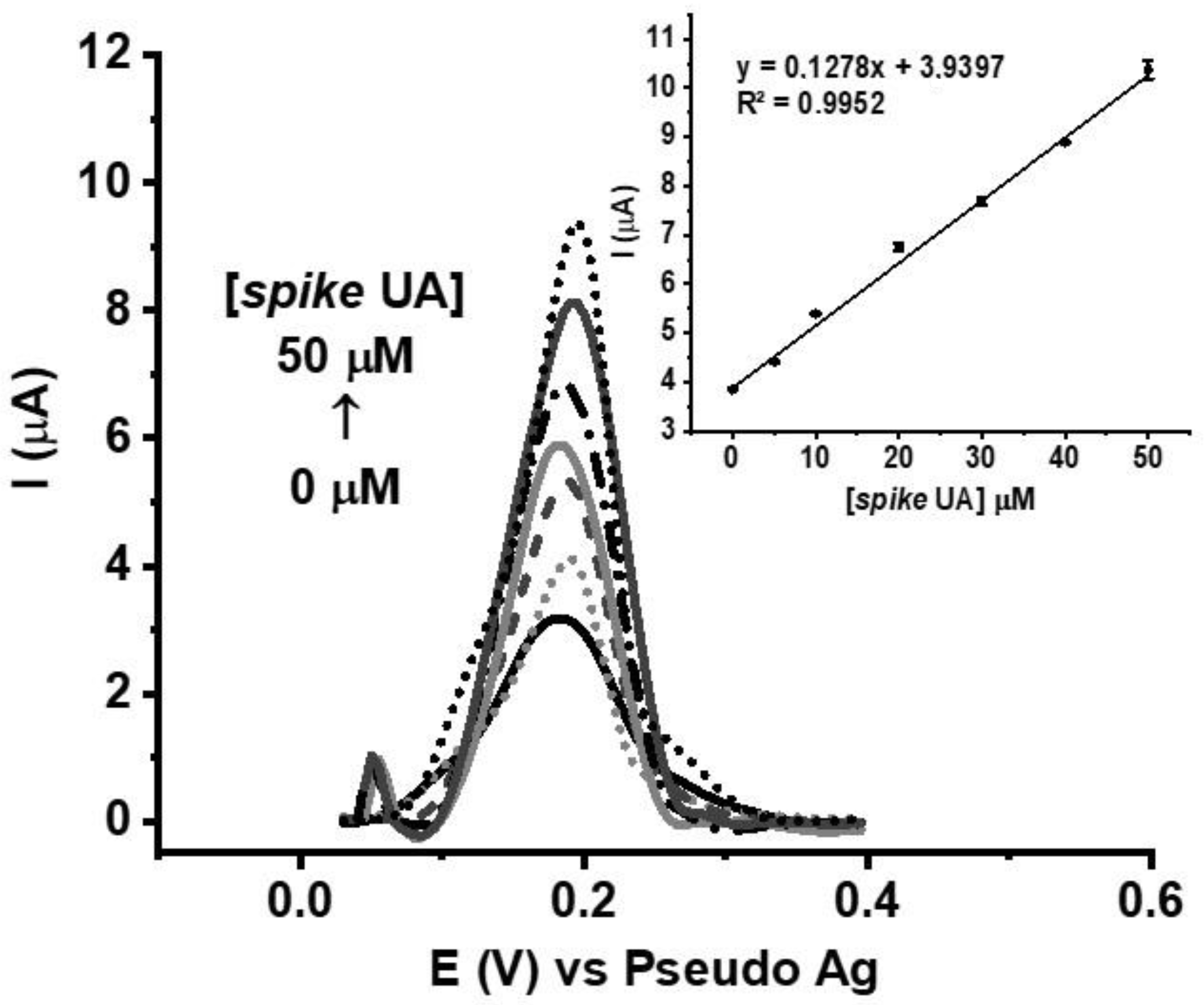
3.4. Analytical Performance of SPE-Based GO/AuNR
3.5. Analysis of Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moravcik, O.; Dvorak, M.; Kuban, P. Autonomous capillary electrophoresis processing and analysis of dried blood spots for high-throughput determination of uric acid. Anal. Chim. Acta 2023, 1267, 341390. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wen, X.; Kong, J. Recent progress on uric acid detection: A review. Crit. Rev. Anal. Chem. 2020, 50, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Verman, G.; Singhai, S.; Rai, P.K.; Gupta, A. A simple approach to develop a paper-based biosensor for real-time uric acid detection. Anal. Methods 2023, 15, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, W.T.; Putra, B.R.; Heryanto, R.; Rohaeti, E.; Yanto, D.H.Y.; Fauzi, A. A simple approach to fabricate a screen-printed electrode and its application for uric acid detection. Int. J. Electrochem. Sci. 2021, 16, 210221. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, J.; Zheng, X.; Shi, C.; Yang, H. Screen printing of stretchable silver nanomaterial inks for a stable human-machine interface. J. Mater. Chem. C. 2023, 11, 5009–5017. [Google Scholar] [CrossRef]
- Anindya, W.; Wahyuni, W.T.; Rafi, M.; Putra, B.R. Electrochemical sensor based on graphene oxide/PEDOT:PSS composite modified glassy carbon electrode for environmental nitrite detection. Int. J. Electrochem. Sci. 2023, 18, 100034. [Google Scholar] [CrossRef]
- Husna, R.; Kurup, C.P.; Ansari, M.A.; Mohd-Naim, N.F.; Ahmed, M.U. An electrochemical aptasensor based on AuNRs/AuNWs for sensitive detection of apolipoprotein A-1 (ApoA1) from human serum. RSC Adv. 2023, 13, 3890–3898. [Google Scholar] [CrossRef]
- Akhavan, O.; Bijanzad, K.; Mirsepah, A. Synthesis of graphene from natural and industrial carbonaceous waste. RSC Adv. 2014, 4, 20441–20448. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2002, 15, 1957–1962. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Braga, G.B.; Tarley, C.R.T.; Pereira, A.C. Thermally reduced graphene oxide: Synthesis, studies and characterization. J. Mater. Sci. 2018, 53, 12005–12015. [Google Scholar] [CrossRef]
- Ossonon, B.D.; Belanger, D. Synthesis and characterization of sulfophenyl-functionalized reduced graphene oxide sheets. RSC Adv. 2017, 7, 27224–27234. [Google Scholar] [CrossRef]
- Khine, Y.Y.; Wen, X.; Jin, X.; Foller, T.; Joshi, R. Functional groups in graphene oxide. Phys. Chem. Chem. Phys. 2022, 24, 26337–26355. [Google Scholar] [CrossRef] [PubMed]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Chrubasik Gryglewicz, G. Characterization of graphite oxide and reduced graphene oxide obtained from different graphite precursors and oxidized by different methods using Raman spectroscopy. Materials 2018, 11, 1050. [Google Scholar] [CrossRef]
- Vecera, P.; Eigler, S.; Kolesnik-Gray, M.; Krstic Vierck, A.; Maultzsch, J.; Schafer, R.A.; Hauke, F.; Hirsch, A. Degree of functionalization dependence of individual Raman intensities in covalent graphene derivatives. Sci. Rep. 2017, 7, 45165. [Google Scholar] [CrossRef]
- Larraza, I.; Ugarte, L.; Fayanas, A.; Gabilondo, N.; Arbelaiz, A.; Corcuera, M.A.; Eceiza, A. Influence of process parameters in graphene oxide obtention on the properties of mechanically strong alginate nanocomposites. Materials 2020, 13, 1081. [Google Scholar] [CrossRef] [PubMed]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified Hummers approach. Int. J. Renew. Energy Environ. 2014, 2, 58–63. [Google Scholar]
- Li, J.; Yan, Q.; Zhang, X.; Zhang, J.; Cai, Z. Efficient conversion of lignin waste to high value bio-graphene oxide nanomaterials. Polymers 2019, 11, 623. [Google Scholar] [CrossRef]
- Li, Y.-L.; Guo, X.; Feng, X.-J.; Li, L.-H. Graphene oxide for inkjet-printing technology. Appl. Mech. Mater. 2015, 748, 77–80. [Google Scholar] [CrossRef]
- Li, P.; Cheng-An, T.; Wang, B.; Huang, J.; Li, T.; Wang, J. Preparation of graphene oxide-based ink for inkjet printing. J. Nanosci. Nnaotechnol. 2018, 18, 713–718. [Google Scholar] [CrossRef]
- Molazemhosseini, A.; Magagnin, L.; Vena, P.; Liu, C.-C. Single-use disposable electrochemical label-free immunosensor for detection of glycated hemoglobin (HbA1c) using differential pulse voltammetry (DPV). Sensors 2016, 16, 1024. [Google Scholar] [CrossRef]
- Safitri, H.; Wahyuni, W.T.; Rohaeti, E.; Khalil, M.; Marken, F. Optimization of uric acid detection with Au nanorod-decorated graphene oxide (GO/AuNR) using response surface methodology. RSC Adv. 2022, 12, 25269–25278. [Google Scholar] [CrossRef]
- Azimzadeh, M.; Rahaie, M.; Nasirizadeh, N.; Ashtari, K.; Naderi-Manesh, H. An electrochemical nanobiosensor for plasma miRNA-155, based on graphene oxide and gold nanorod, for early detection of breast cancer. Biosens. Bioelectron. 2016, 77, 99–106. [Google Scholar] [CrossRef]
- Shanmugam, R.; Aniruthan, S.; Yamunadevi, V.; Nellaiappan, S.; Amali, A.J.; Suresh, D. Co-N/Zn@NPC derived from bimetallic zeolitic imidazolate frameworks: A dual mode simultaneous electrochemical sensor for uric acid and ascorbic acid. Surf. Interfaces 2023, 40, 103103. [Google Scholar] [CrossRef]
- Muhammad, F.; Dik, G.; Kolak, S.; Gedik, K.K.; Bakar, B.; Ulu, A.; Ates, B. Design of highly selective, and sensitive screen-printed electrochemical sensor for detection of uric acid with uricase immobilized polycaprolactone/polyethylene imine electrospun nanofiber. Electrochim. Acta 2023, 439, 141675. [Google Scholar] [CrossRef]
- Reddy, Y.V.M.; Sravani, B.; Agarwal, S.; Gupta, V.K.; Madhavi, G. Electrochemical sensor for detection of uric acid in the presence of ascorbic acid and dopamine using the poly(DPA)/SiO2@Fe3O4 modified carbon paste electrode. J. Electroanal. Chem. 2018, 820, 168–175. [Google Scholar] [CrossRef]
- Soleh, A.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Novel electrochemical sensor using a dual-working electrode system for simultaneous determination of glucose, uric acid, and dopamine. Microchem. J. 2020, 153, 105479. [Google Scholar] [CrossRef]
- Charithra, M.M.; Manjunatha, J.G.G.; Raril, C. Surfactant modified graphite paste electrode as an electrochemical sensor for the enhanced voltammetric detection of estriol with dopamine and uric acid. Adv. Pharm. Bull. 2020, 10, 247–253. [Google Scholar] [CrossRef]
- Putra, B.R.; Nisa, U.; Heryanto, R.; Rohaeti, E.; Khalil, M.; Izzataddini, A.; Wahyuni, W.T. A facile electrochemical sensor based on a composite of electrochemically reduced graphene oxide and a PEDOT:PSS modified glassy carbon electrode for uric acid detection. Anal. Sci. 2022, 38, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Mazzara, F.; Patella, B.; Aiello, G.; O’Riordan, A.; Torino, C.; Vilasi, A.; Inguanta, R. Electrochemical detection of uric acid and ascorbic acid using r-GO/NPs based sensors. Electrochim. Acta 2021, 388, 138652. [Google Scholar] [CrossRef]
- Sohouli, E.; Khosrowshahi, E.M.; Radi, P.; Naghian, E.; Rahimi-Nasrabadi, M.; Ahmadi, F. Electrochemical sensor based on modified methylcellulose by graphene oxide and Fe3O4 nanoparticles: Application in the analysis of uric acid content in urine. J. Electroanal. Chem. 2020, 877, 114503. [Google Scholar] [CrossRef]
| Electrode | Linear Range (μM) | LOD (μM) | LOQ (μM) | Ref. |
|---|---|---|---|---|
| Co-N/Zn@NPC 1 | 0.1–14.7 | 5 × 10−4 | 1.6 × 10−3 | [23] |
| PCL/PEI/UOx/QD 2 | 5–52.0 | 3.96 | 13.1 | [24] |
| Poly(DPA 3) SiO2@Fe3O4 | 1.2–1.8 | 0.4 | 1.2 | [25] |
| Chi/GOx/PB-G 4 | 10–30 | 0.11 | 0.38 | [26] |
| OXL-9 5 | 40–120 | 1.4 | 4.7 | [27] |
| ErGO/PEDOT:PSS 6 | 10–100 | 1.08 | 3.61 | [28] |
| rGO/AuNPs | 10–500 | 3.6 | 10.95 | [29] |
| MC-GO 7-Fe3O4 | 0.5–140 | 0.17 | 0.5 | [30] |
| GO/AuNR | 0.8–200 | 0.5 | 1.0 | This work |
| Interference | The Concentration Ratio (Uric Acid/Interference) | Iox Uric Acid (μA) | % Recovery |
|---|---|---|---|
| - | - | 7.162 ± 0.2 | - |
| Ascorbic acid | 1:1 | 7.855 ± 0.8 | 109.7 ± 1.9 |
| Dopamine | 1:1 | 6.789 ± 0.7 | 94.8 ± 12.6 |
| Glucose | 1:1 | 6.997 ± 0.8 | 97.7 ± 13.6 |
| Urea | 1:1 | 6.902 ± 0.1 | 96.4 ± 3.6 |
| Sodium | 1:1 | 7.893 ± 0.2 | 110.2 ± 5.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahyuni, W.T.; Safitri, H.; Rohaeti, E.; Khalil, M.; Putra, B.R. A Novel Approach to Fabricating a Screen-Printed Electrode Based on a Gold Nanorod–Graphene Oxide Composite for the Detection of Uric Acid. Eng. Proc. 2023, 48, 24. https://doi.org/10.3390/CSAC2023-14908
Wahyuni WT, Safitri H, Rohaeti E, Khalil M, Putra BR. A Novel Approach to Fabricating a Screen-Printed Electrode Based on a Gold Nanorod–Graphene Oxide Composite for the Detection of Uric Acid. Engineering Proceedings. 2023; 48(1):24. https://doi.org/10.3390/CSAC2023-14908
Chicago/Turabian StyleWahyuni, Wulan Tri, Hana Safitri, Eti Rohaeti, Munawar Khalil, and Budi Riza Putra. 2023. "A Novel Approach to Fabricating a Screen-Printed Electrode Based on a Gold Nanorod–Graphene Oxide Composite for the Detection of Uric Acid" Engineering Proceedings 48, no. 1: 24. https://doi.org/10.3390/CSAC2023-14908
APA StyleWahyuni, W. T., Safitri, H., Rohaeti, E., Khalil, M., & Putra, B. R. (2023). A Novel Approach to Fabricating a Screen-Printed Electrode Based on a Gold Nanorod–Graphene Oxide Composite for the Detection of Uric Acid. Engineering Proceedings, 48(1), 24. https://doi.org/10.3390/CSAC2023-14908






