Abstract
Hydrogen peroxide (H2O2) is an aqueous solution that is widely used for oxidation, disinfection and sterilization, and its detection is very important in the fields of biological health and environment. The main detection methods of H2O2 include colorimetric, electrochemical, enzymatic and fluorescence analysis. However, due to the influence of moisture and oxidation, it is very difficult to realize simple, convenient, real-time and efficient detection technology for hydrogen peroxide vapor (HPV). Recently, our group proposed adding ammonium titanyl oxalate (ATO) to the sensing film composite system to prepare a chemosensor based on PEDOT:PSS-ATO/PEDOT composite film. The limit of detection (LOD) of the film was 1.0 ppm, and the linear trend was in the range of 1.0 ppm to 10.5 ppm. We then explored the influence of various material systems on its HPV sensing performance, which exhibited both electrical and colorimetric responses. This study was expected to realize a practical HPV sensor as well as promote the further application of PEDOT-based composites in the field of chemosensors.
1. Introduction
Hydrogen peroxide (H2O2) aqueous solution is an environmentally friendly and efficient oxidant and disinfectant. The outbreak of the novel coronavirus has led to the strengthening of disinfection and sterilization measures in public facilities, transportation, hospitals, nursing homes, and even ordinary families all over the world [1,2]. However, its widespread use is hazardous. For example, the strong oxidative properties of H2O2 may cause corrosion and irritation to the skin, eyes and mucous membranes of the human body. If the solution enters the human body, oxidative stress reactions may occur to generate free radicals, resulting in cell damage and some diseases, as well as accelerating the growth of the human body. Therefore, in view of the importance of biological and environmental safety, it is very important to develop effective detection technologies for H2O2.
At present, most of the research on H2O2 sensors focused on its detection in liquid phase systems [3]. In view of its wide use as environmental disinfectants and explosive markers, the importance of H2O2 vapor (HPV) monitoring in the air also cannot be ignored. In most cases, the HPV detection begins by firstly making it cool and adsorptive in water, and then using electrochemical methods to measure the liquid concentration [4]. In addition, the detection of HPV can also be achieved via fluorescence analysis [5,6], colorimetry [7], etc. Fluorescence methods generally have high sensitivity and selectivity, but there is a problem related to cumbersome material synthesis. In comparison, the colorimetric method is simple, fast and direct, and is seen as an important direction for sensor popularization research. On the other hand, the chemiresistive sensor is often used to monitor humidity and various gases, and has the advantages of low cost, small size, easy use, facile to construct device and real-time monitoring.
To explore effective HPV sensing materials, recently, our group investigated the applicability of poly(3,4-ethylenedioxythiophene) (PEDOT)-based chemiresistive and colorimetric dual-mode sensors [8,9]. Among them, poly(3,4-ethylenedioxythiophene)–polystyrene sulfonate (PEDOT:PSS)–ammonium titanyl oxalate (ATO)/PEDOT composite film was demonstrated as a good platform for highly selective, sensitive and naked-eye HPV sensing. The system was designed for the following reasons [5].
On one hand, intrinsically conductive polymers (ICPs), especially those in the PEDOT family, are considered to be important and good candidate sensing materials [10]. Their unique tunable electrical properties, as well as their advantages of easy synthesis, structural diversity, functionalization, and flexibility, make them versatile in sensor applications. At present, the application of PEDOT in this field has developed into many types, such as electrochemistry, chemoresistance, piezoelectricity, etc. In particular, their unique doping/dedoping process makes them one of the most promising candidates for chemistoric sensor applications for gas detection at room temperature [11]. PEDOT:PSS has special advantages [12], and has also been studied in relation to electrochemical detection of H2O2 in liquid phase system, and to evaluate its chemistoric response to many gas phase analytes like NH3, CO, NOx, etc.
On the other hand, the hygroscopicity of PEDOT:PSS film itself is conducive to the adsorption of HPV, and the electrical and optical properties will be affected by the oxidation of HPV. Additionally, the hydrophobic PEDOT outer layer could improve the film surface roughness and weaken its hygroscopicity, which will help maintain its stability and sensing accuracy, especially in high humidity.
Moreover, in view of the fact that, HPV is difficult to distinguish by smell and vision due to its colorless appearance and light odor, ATO, as a colorless compound that has characteristic complexation-induced discoloration when mixed with H2O2, was introduced into the gas-sensitive material system. It can basically eliminate the interference of all coexisting gases in the normal environment and thus ensure high selectivity [5]. At the same time, the color change reaction will provide a colorimetric sensing signal, which enhances the signal readability.
2. Materials and Methods
In this work, the PEDOT:PSS/ATO dispersion was spin-coated on the ITO conducting glass and dried in an oven at 50 °C to prepare the PEDOT:PSS-ATO film. The electrochemical correlation tests were performed using a three-electrode system. The well-prepared PEDOT:PSS-ATO film was selected as the working electrode, the platinum sheet as the counter electrode, Ag/AgCl as the reference electrode, and acetonitrile/tetrabutylammonium hexafluorophosphate (0.1 M) as the electrolyte and EDOT (1 mM) was added in as a unit of electropolymerization. The PEDOT layer with a certain amount of polymerization charge was electrodeposited on the surface of the composite film via electrochemical polymerization, and the PEDOT:PSS-ATO/PEDOT composite film was prepared. After that, we explored the resistance response and colorimetric response behavior and effect of the composite film under different concentrations of HPV.
3. Results and Discussion
The PEDOT:PSS-ATO and PEDOT:PSS-ATO/PEDOT composite films were prepared via conventional mechanical blending and electrochemical methods [8,9].
As seen in Figure 1a, the PEDOT:PSS-ATO film exhibited a typical moisture response of PEDOT:PSS film. As the humidity increased, the resistance value reached the maximum value (about 20 min, 7.6 times of the initial value) at about 50~60% RH. And then with the increases in the humidity, the resistance of film continued to decrease until it fell to the same level as the initial value. The resistance response curve (Figure 1b) of the PEDOT:PSS-ATO/PEDOT composite film was similar to that of the PEDOT:PSS/PEDOT film [8]. The resistance of the PEDOT:PSS-ATO/PEDOT film increased rapidly from the initial value (about 53 Ω, the PEDOT:PSS/PEDOT film was about 48 Ω [8]) and decreased rapidly until it reached levels similar to its initial values after 20 min. The resistance response values (ΔR/R0) calculated based on the peak value were 5.68 (10.5 ppm), 4.42 (4.0 ppm), 2.19 (1.9 ppm), 0.30 (1.0 ppm), respectively, and the time taken to reach the peak value was 14 min, 13 min, 9 min, and 10 min [9]. Compared with our previous report, the reaction of ATO with H2O2 consumed part of HPV while generating the H2O, thus speeding up the entire process of HPV film detection. Under the coverage of the PEDOT layer, the H2O generated from the reaction of ATO and H2O2 was locked in the PEDOT:PSS-ATO layer, which accelerated the detection process. All samples were tested in parallel three times and their average values were taken. The resistance response-HPV concentration data of the PEDOT:PSS-ATO/PEDOT composite film were fitted using the ExpAssoc function. The final fitting curve is shown in Figure 1b (inset), y = 5.75634 × (1 − e(−x/2.42891)), R2 = 0.85399.
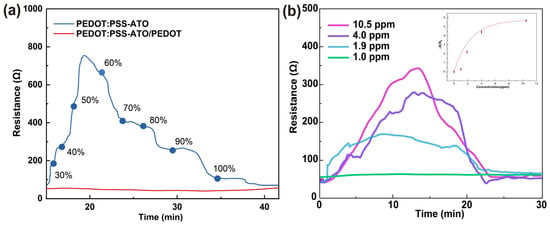
Figure 1.
(a) PEDOT:PSS-ATO and PEDOT:PSS-ATO/PEDOT films under increasing levels of ambient humidity (30~100% RH); (b) resistance–time curve of PEDOT:PSS-ATO/PEDOT film (Inset: resistance response-HPV concentration fitting curve of PEDOT:PSS-ATO/PEDOT film).
Here, we evaluate the colorimetric sensing based on the ΔE value. ΔE is the comprehensive total color difference, which can be calculated using the formula:
where ΔL, Δa, and Δb are the effects of PEDOT-based composite film on HPV. The colorimetric signal response to the color difference test results can be tested and recorded using a portable color difference meter [9]. According to the results shown in Figure 2, the ΔE value [9] of PEDOT:PSS film increased with the increase in HPV concentration, even at low concentrations (1.0 ppm), and subsequently, a slight color difference could be observed with the naked eye (ΔE = 2.11). However, when PEDOT was deposited on the PEDOT:PSS film, with the increase in the HPV concentration, the color change after the detection of HPV on the composite film was not obvious, and only when the original composite film was compared with the composite film that had detected HPV concentrations of 10.5 ppm could the naked eye slightly detect the color change (ΔE = 2.08). The PEDOT layer on the surface can not only slow down the immersion of moisture, but also hinders the entry of H2O2 components, which weakens the colorimetric response generated by the interaction between PEDOT:PSS layer and H2O2 components to a certain extent. The oxidative color-changing substance ATO, which responded characteristically to H2O2, was added to the PEDOT:PSS aqueous dispersion through mechanical blending, and PEDOT:PSS-ATO and PEDOT:PSS-ATO/PEDOT composite films were prepared. With the introduction of HPV and the increase in the concentration, the two composite films were observed to change from blue to yellow-green. Since ATO can react with H2O2 in a highly selective color change, compared with the monotonous blue shades of PEDOT:PSS and PEDOT, the detection of HPV by PEDOT:PSS-ATO and PEDOT:PSS-ATO/PEDOT composite films presents a richer and more obvious color change signal, so that the naked eye can more clearly identify the working condition of the sensor, and at a 1.0 ppm concentration of HPV, the ΔE value of PEDOT:PSS-ATO composite films reached 2.6. In the 4.0 ppm and 10.5 ppm concentration of HPV, the film color was obviously yellowish green. Compared with the colorimetric response of HPV detected by PEDOT:PSS/PEDOT composite film, the ΔE values of PEDOT:PSS-ATO/PEDOT composite films are higher. When the concentration of HPV is 1.0 ppm, the color change of the film can be slightly detected (ΔE = 1.57). Colorimetric response increased by nearly 128% at the same concentration, and the color change under the naked eye was more significant.
ΔE = [(ΔL)2 + (Δa)2 + (Δb)2]1/2
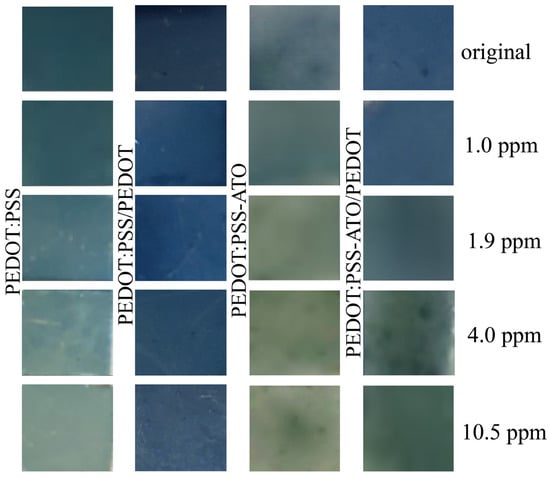
Figure 2.
Photos of PEDOT, PEDOT:PSS, PEDOT:PSS-ATO and PEDOT:PSS-ATO/PEDOT films before and after testing of different concentrations of HPV.
The paper-based colorimetric sensing system [5] was first described in 2011 by Prof. Zang Ling’s research group, which has made breakthrough contributions to HPV detection. This system can detect hydrogen peroxide vapor exceeding a few parts per billion, with a detection limit of 4.3 ppm. When titanium-based peroxide test paper [13] was exposed to peroxide vapor in the peroxide concentration range from 2.0 to 30.0 ppm, first-order behavior of color change with time was observed, and the detection limit was as low as 1.0 ppm. Comparing the linear range and limit of determination (LOD) with other sensors for hydrogen peroxide detection (Table 1), it can be seen that the PEDOT:PSS-ATO/PEDOT film sensor has a lower detection limit and can achieve low-concentration target analysis object detection. Different from liquid phase detection, research on HPV detection is lacking. Our work not only studies the effect of applying PEDOT:PSS-based gas-sensing materials to HPV varistor sensing, but also enhances the effect of colorimetric signal detection. The future optimization of the PEDOT:PSS-based composite system using the dual-mode detection mechanism provides theoretical support.

Table 1.
Comparison of detection linear range and detection limit of sensors for hydrogen peroxide vapor detection.
4. Conclusions
In summary, ATO was added to the PEDOT:PSS aqueous dispersion to prepare PEDOT:PSS-ATO film and then EDOT was electrochemically polymerized at its surface, forming PEDOT:PSS-ATO/PEDOT composite film. It was successfully used for chemiresistive and colorimetric detection of HPV. Since ATO can react with H2O2 in a highly selective color change, compared with the monotonous blue shade change of PEDOT:PSS and PEDOT films, the detection of HPV by these films showed a much richer and more pronounced color change signal, allowing naked-eye identification. In particular, PEDOT:PSS-ATO/PEDOT film can detect HPV at a low concentration of 1.0 ppm via dual modes, which provides its application prospect for practical HPV monitoring under the safety threshold.
Author Contributions
Conceptualization, S.C; methodology, S.C. and N.G.; software, S.A., N.G. and X.X.; validation, X.X. and N.G.; formal analysis, S.A.; investigation, N.G. and S.C.; resources, S.C.; data curation, N.G.; writing—original draft preparation, S.A., N.G. and X.X.; writing—review and editing, S.C.; supervision, S.C.; project administration, S.C.; funding acquisition, S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Academic Development Project of TongXin Funds (grant number 2023161808).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berger, D.; Gundermann, G.; Sinha, A.; Moroi, M.; Goyal, N.; Tsai, A. Review of aerosolized hydrogen peroxide, vaporized hydrogen peroxide, and hydrogen peroxide gas plasma in the decontamination of filtering facepiece respirators. Am. J. Infect. Control 2022, 50, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Dewey, H.M.; Jones, J.M.; Keating, M.R.; Budhathoki-Uprety, J. Increased use of disinfectants during the COVID-19 pandemic and its potential impacts on health and safety. ACS Chem. Health Saf. 2022, 29, 27–38. [Google Scholar] [CrossRef]
- Pashkova, A.; Svajda, K.; Black, G.; Dittmeyer, R. Automated system for spectrophotometric detection of liquid phase hydrogen peroxide for concentrations up to 5% w/w. Rev. Sci. Instrum. 2009, 80, 055104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; Zhou, S.; Zhang, Y.J. Ultrasensitive detection of hydrogen peroxide using Bi2Te3 electrochemical sensors. ACS Appl. Mater. Interfaces 2021, 13, 4761–4767. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Bunes, B.R.; Zang, L. Paper-based vapor detection of hydrogen peroxide: Colorimetric sensing with tunable interface. ACS Appl. Mater. Interfaces 2011, 3, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Feng, Y.T.; Zhang, Z.X.; Zhang, M. Highly efficient fluorescent film probe of hydrogen peroxide vapor. Microchem. J. 2020, 158, 105290. [Google Scholar] [CrossRef]
- Tran, H.V.; Nguyen, T.V.; Nguyen, L.T.N.; Hoang, H.S.; Huynh, C.D. Silver nanoparticles as a bifunctional probe for label-free and reagentless colorimetric hydrogen peroxide chemosensor and cholesterol biosensor. J. Sci. Adv. Mater. Devices 2020, 5, 385–391. [Google Scholar] [CrossRef]
- Xie, X.W.; Gao, N.; Zhu, L.; Hunter, M.; Chen, S.; Zang, L. PEDOT:PSS/PEDOT film chemiresistive sensors for hydrogen peroxide vapor detection under ambient conditions. Chemosensors 2023, 11, 124. [Google Scholar] [CrossRef]
- Xie, X.W.; Gao, N.; Hunter, M.; Zhu, L.; Yang, X.M.; Chen, S.; Zang, L. PEDOT films doped with titanyl oxalate as chemiresistive and colorimetric dual-mode sensors for the detection of hydrogen peroxide vapor. Sensors 2023, 23, 3120. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Z.; Ishak, K.M.K.; Zawawi, M.A.M.; Zulkifli, Z.; Jaafar, M.; Ahmad, Z. Single-step treatment to improve conductivity of PEDOT:PSS by hydrobromic acid solution for application of transparent electrode. Org. Electron. 2022, 110, 106643. [Google Scholar] [CrossRef]
- Gao, N.; Yu, J.R.; Tian, Q.Y.; Shi, J.F.; Zhang, M.; Chen, S.; Zang, L. Application of PEDOT:PSS and its composites in electrochemical and electronic chemosensors. Chemosensors 2021, 9, 79. [Google Scholar] [CrossRef]
- Vigna, L.; Verna, A.; Marasso, S.L.; Sangermano, M.; D’Angelo, P.; Pirri, F.C.; Cocuzza, M. The effects of secondary doping on ink-jet printed PEDOT:PSS gas sensors for VOCs and NO2 detection. Sens. Actuators B Chem. 2021, 345, 130381. [Google Scholar] [CrossRef]
- Hossain, R.; Dickinson, J.J.; Apblett, A.; Materer, N.F. Detection of Hydrogen Peroxide in Liquid and Vapors Using Titanium(IV)-Based Test Strips and Low-Cost Hardware. Sensors 2022, 22, 6635. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).