1. Introduction
X-ray microtomography is one of the non-destructive techniques which is increasingly used in many scientific areas including biomedical and material research and industrial R&D, as well as drug quality and control processes [
1,
2,
3]. Combining this method of analysis with the Bruggeman model, Markl et al. [
4] obtained new data on the shape and orientation of the pores in tablets with calcium carbonate. The authors demonstrated that intermolecular pores were the main reason for the anisotropic behavior of the medium [
4]. Both the presence and the structure of the pores affects the way the liquid penetrates into the tablet, which translates into a distribution of the rate of pharmacological effect [
4]. The microtomography method was also used to detect fibers in nanoscale samples of electrospun poly(caprolactone)-based materials [
5]. In turn, Holm et al. [
6] used microtomograhy to examine the effect of microwave radiation on the internal structure, as well as strength parameters, and the disintegration behavior of multiparticulate drug delivery systems.
The present study aimed to assess the stability of effervescent solid dosage forms using a non-invasive X-ray microtomography technique.
2. Methods
We selected effervescent tablets which contained vitamin C and are popular on the Polish market. One type of the tablets was stored according to the manufacturer’s instructions for several years until the expiration date (expired tablets with expiration date: February 2020), and the other type of tablets was within the expiry date (unexpired tablets with expiration date: April 2023).
The basic parameters of the tablets, i.e., weight, diameter, thickness, and disintegration time, as well as features of the mechanical strength, were assessed according to Pharmacopeia European [
7].
The scanning analysis of the selected preparations was completed using an X-ray microtomography apparatus (GE Sensing & Inspection Technologies GmbH, Wunstorf, Germany). The voltage at which the tablets were scanned was 180 kV. An object analyzed with this method absorbs X-rays proportionally to its density. In the microtomographic image, the density is reflected by the level of gray, i.e., high-density areas are represented by bright pixels, whereas low-density areas are dark. To establish dependence between the density and the brightness of the pixels, we fixed the tablets onto a special phantom with areas of known density (Micro-CT HA Phantom D32). In this way, we kept the same analysis conditions for both the phantom and analyzed preparations.
All obtained data were analyzed with the use of the Statistica 13 software (STATSOFT; Statistica, Tulsa, OK, USA). A U Mann–Whitney non-parametric test was used to compare obtained data between expired and unexpired tablets due to the preliminary nature of the research. The results were considered significant at a p-value of ≤0.05.
3. Results and Discussion
The stability of the finished medicinal product has a significant impact on the effectiveness of the medicinal product, as well as the safety of the patient. With this in mind, stability testing is now a legal requirement for medicinal products throughout the drug development process. Several novel methods were proposed to evaluate the drug forms after storage in both ambient conditions and stressful conditions. X-ray microtomography allows for the 3D characterization of the particle structure and, in particular, the solid dosage forms. Furthermore, determining the distribution of active pharmaceutical ingredients (API) in pharmaceutical tablets allows for the optimization of the formulations of these drug forms. Wagner-Hattler et al. [
8] used synchrotron X-ray microtomography to assess the uniformity of the distribution of moxidectin in minitablets. Drug loading of up to 20% has been shown not to segregate moxidectin [
8]. In our previous study, microtomographic analysis was used i.a. to assess the porosity of the effervescent preparation with magnesium and vitamin B6 [
9]. In this study, we also used expired tablets, which had a larger pore diameter and a higher percentage of porosity than tablets tested before the expiration date.
3.1. Basic Parameters of the Analyzed Tablets
The tested tablets were cylindrical with flat top and side surfaces. We observed differences in the weight, thickness, and diameter between unexpired and expired tablets. The disintegration time of expired tablets was significantly shorter than the disintegration time of unexpired tablets (1.37 min vs. 1.44 min, p = 0.005). In addition, expired tablets showed significantly lower mechanical strength compared to unexpired tablets.
The moisture content was 0.4% and 0.2% for expired and unexpired tablets, respectively.
3.2. Microtomography Analysis
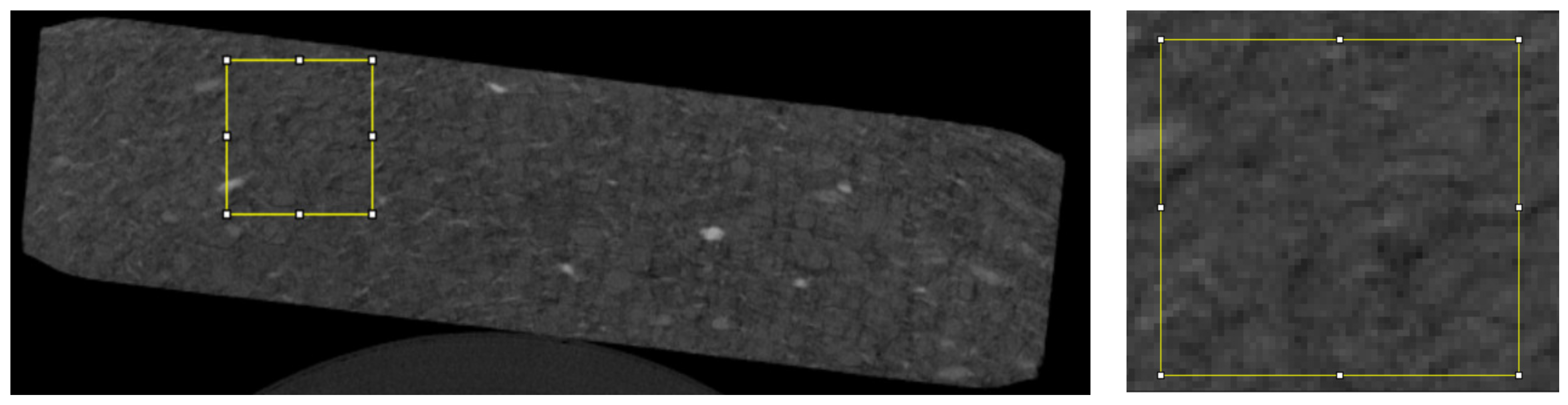
For the analysis, we used 20 randomly selected microtomographic slices of each type of tablet in which a total of 70 regions of interest (ROIs) were evaluated (
Figure 1).
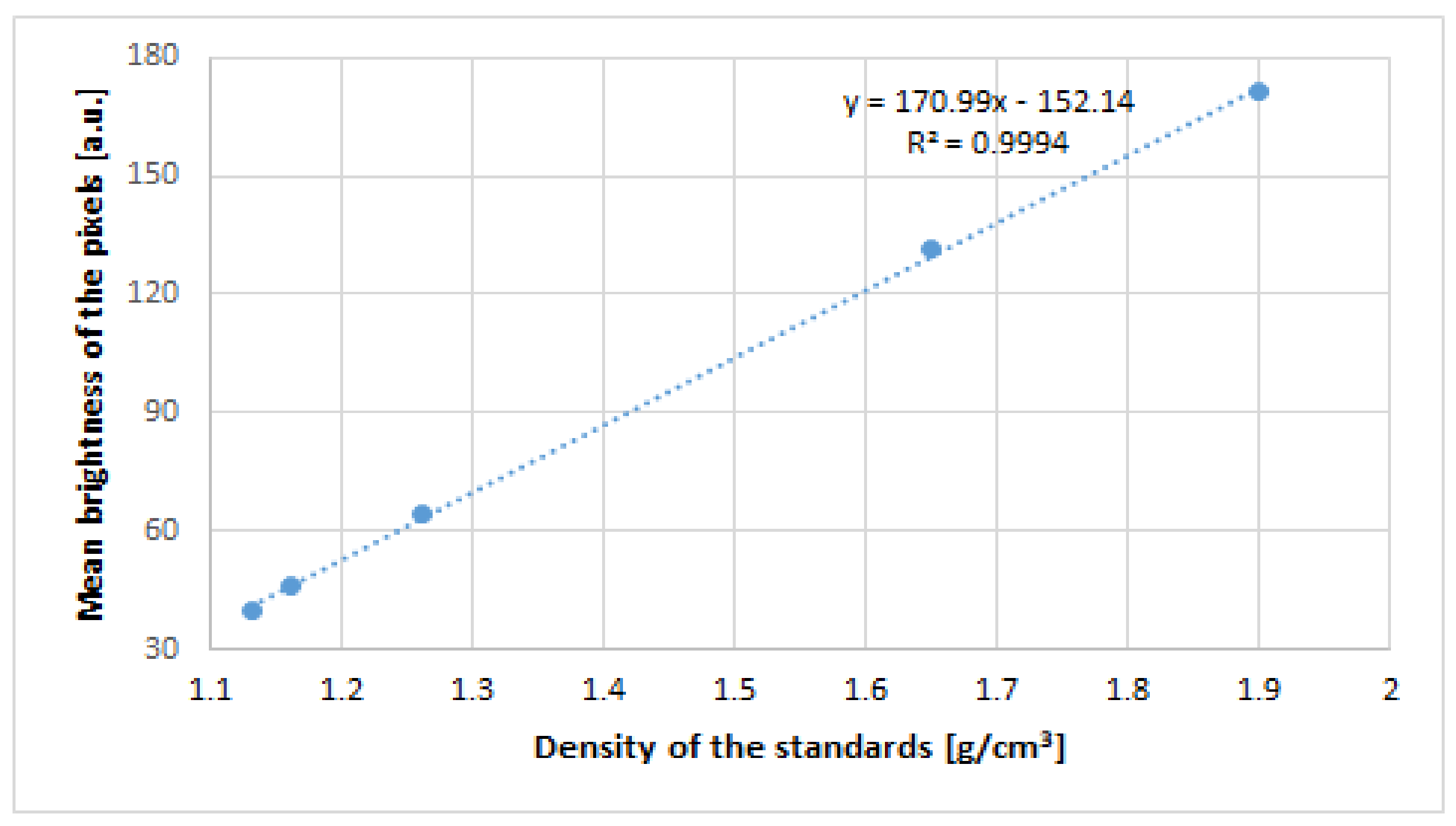
The average brightness of the pixels was measured with ImageJ software (ImageJ 1.53a; National Institutes of Health, Madison, WI, USA). A curve of dependence between the brightness and density of the known area from the phantom was drawn (
Figure 2).
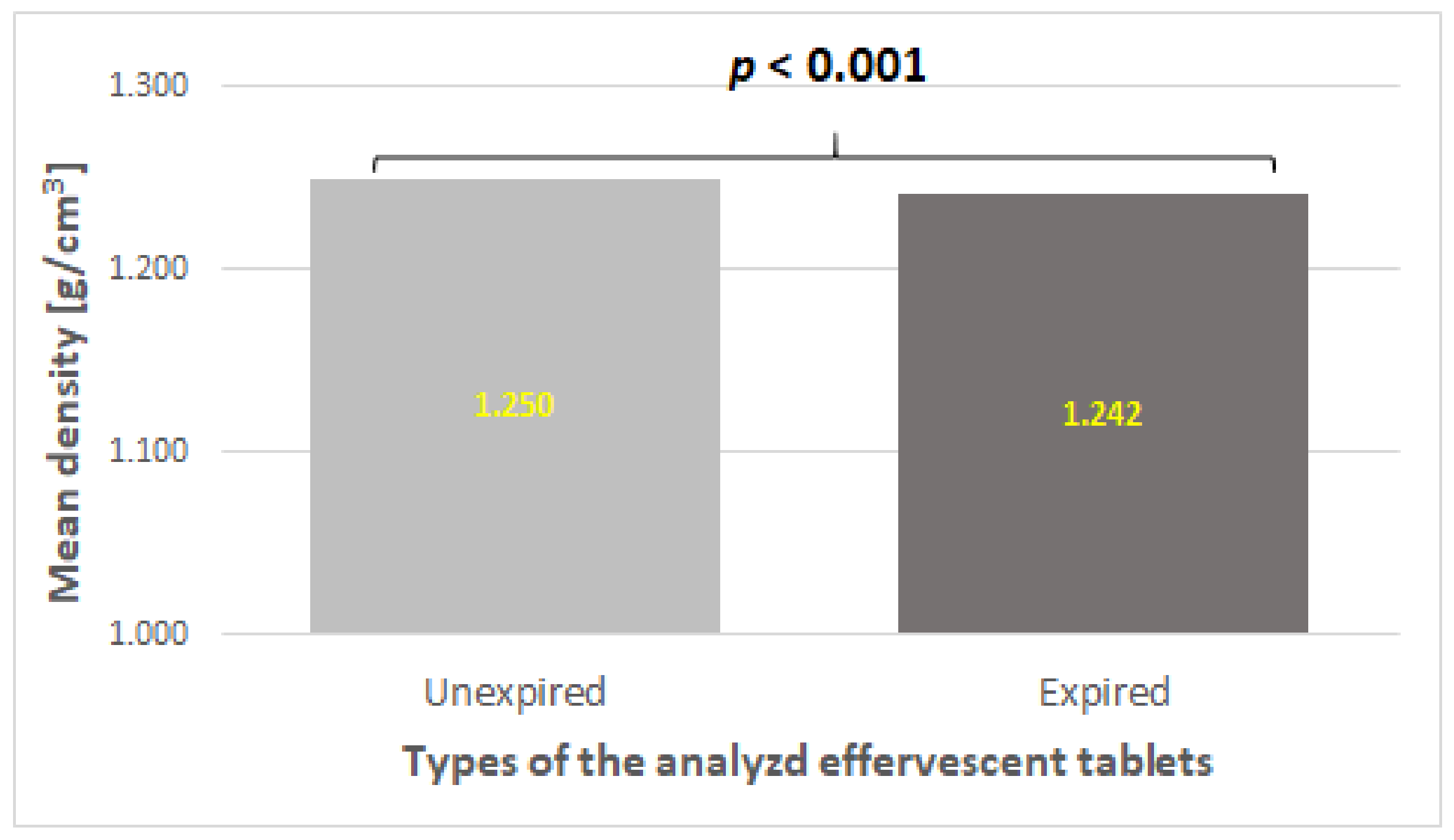
The density of the inner structure differed significantly between the two types of studied effervescent tablets with vitamin C (
p < 0.001). Tablets within the expiration date had a higher mean density than tablets outside the expiration date (
Figure 3). This may indicate that unexpired effervescent tablets containing vitamin C showed better homogeneity than expired ones.
4. Conclusions
The applied method of three-dimensional microtomography imaging allowed for the rapid detection of the differences in the microstructure of expired and unexpired tablets. On the basis of quantitative data, significant differences in the tablet density of the analyzed drug forms were demonstrated, which indicated that the homogeneity of the tablets was lower after storage at ambient conditions for a long time.
Author Contributions
Conceptualization, B.S.-H., B.S.-M. and P.D.; methodology, B.S.-H., B.S.-M., M.M. and P.D.; software, B.S.-H. and B.S.-M.; formal analysis, B.S.-H. and B.S.-M.; investigation, B.S.-H., B.S.-M., M.M. and P.D.; data curation, B.S.-H., B.S.-M., M.M. and P.D.; writing—original draft preparation, B.S.-H., B.S.-M., M.M. and P.D.; writing—review and editing, B.S.-H. and B.S.-M.; visualization, B.S.-H.; supervision, B.S.-H.; project administration, B.S.-H.; funding acquisition, B.S.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Silesia in Katowice, Poland, with project no. PCN-1-058/K/2/O.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in the study may be made available upon request after contacting the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sacré, P.Y.; Alaoui Mansouri, M.; De Bleye, C.; Coïc, L.; Hubert, P.; Ziemons, E. Evaluation of distributional homogeneity of pharmaceutical formulation using laser direct infrared imaging. Int. J. Pharm. 2022, 612, 121373. [Google Scholar] [CrossRef]
- Schomberg, A.K.; Diener, A.; Wünsch, I.; Finke, J.H.; Kwade, A. The use of X-ray microtomography to investigate the microstructure of pharmaceutical tablets: Potentials and comparison to common physical methods. Int. J. Pharm. X 2021, 3, 100090. [Google Scholar] [CrossRef] [PubMed]
- Heidarloo, N.; Aghamiri, S.M.R.; Saghamanesh, S.; Azma, Z.; Alaei, P. Generation of material-specific energy deposition kernels for kilovoltage X-ray dose calculations. Med. Phys. 2021, 48, 5423–5439. [Google Scholar] [CrossRef]
- Markl, D.; Wang, P.; Ridgway, C.; Karttunen, A.P.; Chakraborty, M.; Bawuah, P.; Pääkkönen, P.; Gane, P.; Ketolainen, J.; Peiponen, K.E.; et al. Characterization of the Pore Structure of Functionalized Calcium Carbonate Tablets by Terahertz Time-Domain Spectroscopy and X-ray Computed Microtomography. J. Pharm. Sci. 2017, 106, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Chiverton, J.P.; Kao, A.; Roldo, M.; Tozzi, G. Automatic diameter and orientation distribution determination of fibrous materials in micro X-ray CT imaging data. J. Microsc. 2018, 272, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Holm, T.P.; Kokott, M.; Knopp, M.M.; Boyd, B.J.; Berthelsen, R.; Quodbach, J.; Löbmann, K. Development of a multiparticulate drug delivery system for in situ amorphisation. Eur. J. Pharm. Biopharm. 2022, 180, 170–180. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia, 10th ed.; EDQM Council of Europe: Strasbourg, France, 2022.
- Wagner-Hattler, L.; Québatte, G.; Keiser, J.; Schoelkopf, J.; Schlepütz, C.M.; Huwyler, J.; Puchkov, M. Study of drug particle distributions within mini-tablets using synchrotron X-ray microtomography and superpixel image clustering. Int. J. Pharm. 2020, 573, 118827. [Google Scholar] [CrossRef] [PubMed]
- Meisner, M.; Duda, P.; Szulc-Musioł, B.; Sarecka-Hujar, B. Characteristics of Commercial Effervescent Tablets Using Selected Pharmacopeial and Novel Analytical Methods. Appl. Sci. 2023, 13, 3171. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).