Abstract
In this work, a new hydrophobic deep eutectic solvent (HDES) based on trioctylphosphine oxide and di-(2-ethylhexyl)phosphoric acid was obtained. The formation of HDES was confirmed by NMR and FT-IR spectroscopy. The physical properties of the synthesized HDES were studied, density, dynamic viscosity, refractive index in the range of 15–60 °C, and phase diagram. The possibility of extraction of Co(II) and Al(III) ions from nitrate aqueous solutions was studied. The regularities of the interfacial distribution of metal ions were studied depending on the time of the extraction process, the ratio of components in HDES and pH of the aqueous phase. It has been shown that this HDES can be used for the selective extraction of metal ions.
1. Introduction
Nowadays there is a pressing need to recycle LIB, due to their wide and rapidly growing usage and the high content of valuable elements in their composition (for example, Li, Co, Ni, etc.) [1]. The most common, universal, and durable method of separation and concentration of complex mixtures valuable metals is liquid–liquid extraction. Along with the task of increasing the efficiency of the extraction process, there is an equally important task for the implementation of environmental safety for the process. So, the most promising class of solvents for the extraction of metals from aqueous solutions are HDES types of III and V [2]. HDES have a lower melting point compared to the starting components which are a mixture of organic compounds (hydrogen bond acceptor (HBA) and hydrogen bond donor (HBD)). The main advantages of these solvents are ease of preparation, often low cost, availability, and a wide choice of reagents, which makes it possible to vary the physicochemical properties and achieve selectivity to target compounds [3,4,5]. The combination of these and other advantages of HDES makes them one of the most promising extractants, favorably distinguishing them from ionic liquids and classical extractants.
In this paper, HDES based on trioctylphosphine oxide (TOPO) and di-(2-ethylhexyl)phosphoric acid (D2EHPA), as an extractant for Co (II) and Al (III) ions, will be considered. These metals were chosen due to their wide usage in LIB cathode materials; Co is in the composition of the cathode powder, and Al is its substrate [6]. Previously, the TOPO:D2EHPA mixture was considered as a synergistic mixture and was used in organic solvents for the extraction of various metal ions [7,8]. In this work, the above mixture is considered as HDES, the formation of which was confirmed by FT-IR, NMR spectroscopy, and differential scanning calorimetry (DSC).
2. Materials and Methods
D2EHPA (Acros Organics, 95%) and TOPO (Alfa Aesar, 98%) were used without further purification. Aluminum(III) and cobalt(II) nitrates, nitric acid, and sodium hydroxide (Khimmed) were used with >99% purity. All regents were weighed on an AND HR-100AZ analytical balance (Japan).
To form HDES, the reagents were placed in a test tube and mixed at a temperature of 60 °C. The study of physicochemical properties was carried out on the composition D2EHPA:TOPO 8:2, since this composition is eutectic, except for the FT-IR spectra, since the interactions of the components in this case are clearly visible at a composition of 1:1. 31P spectra were recorded in DMSO-d6 on a Bruker Fourier 300 HD spectrometer (USA). IR Fourier spectra in the range 4000–600 cm−1 were recorded on a Shimadzu IRTracer-100 spectrometer (Japan). HDES density was determined on an Anton Paar DMA 1001 instrument (Austria). The refractive index was determined on an Anton Paar Abbemat 3200 refractometer (Austria). Viscosity was studied by rotational rheometry on a Physica MCR301 rheometer (Anton Paar), measuring unit-cone-plane, cone diameter 50 mm, and cone angle 1°. The HDES phase diagram was constructed using the DSC method on a DSK-500 instrument (Russia). For this, at least nine samples were prepared, covering the range of mole fractions from 0.1 to 0.9. Liquid mixtures of 10 mg were introduced into aluminum DSC crucibles and cooled to −130 °C at a rate of 5 °C/min and kept for 10 min, and then heated at a rate of 5 °C/min. When constructing the liquidus line, only those temperatures were taken into account at which the complete disappearance of the solid phase was achieved. The pH value of the aqueous phase before and after extraction was determined using an OHAUS Starter 5000 pH meter (USA) with a STMICRO5 RU combined glass electrode.
All experiments on the study of extraction were carried out until the thermodynamic equilibrium was established. The volume ratio of the aqueous phase and the HDES phase was 5:1, respectively. The initial concentration of metal ions was 0.01 mol/L. The content of Co(II) in the aqueous phase before and after extraction was determined by spectrophotometry in the visible region (λ = 510 nm) using 4-(2-pyridylazo)resorcinol as an indicator, and the concentration of Al(III) was determined using xylenol orange (λ = 555 nm). The concentration of metal ions in the HDES phase was determined from the difference between the concentrations in the initial solution and in the aqueous phase after extraction. Determination of optical density values was carried out on the device Ekroskhim PE-5400UF (Russia) in glass cuvettes l = 10 mm. The error in determining the concentrations was <5%. The presented experimental data are the result of a series of experiments and processed by methods of mathematical statistics.
3. Results and Discussion
3.1. Characterization of D2EHPA-TOPO HDES
Infrared spectroscopy makes it possible to establish the nature of the intermolecular interaction between HDES components. Figure S1 shows the spectra of the starting D2EHPA and TOPO and HDES based on them at a ratio of 1:1. According to Griffiths’s theory [9], the weakening of the hydrogen bonds will increase the electron cloud density and the chemical bond constant of the O–H and P=O bonds, leading the vibration absorption peaks to move to a higher wavenumber.
The spectra of pure D2EHPA and HDES show peak shifts from 2304.94 to 2320.37 cm−1 and from 1224.80 to 1265.30 cm−1, which are characterized by stretching vibrations of O–H and P=O groups, respectively, which indicates weakening of hydrogen bonds in D2EHPA [10], and also on the participation of the O-H group in the formation of a hydrogen bond with the P=O group. The results of the analysis of the FT-IR spectra of HDES compared to the initial TOPO spectra showed that the stretching vibration band of the P=O group in TOPO shifts from 1145.72 to 1114.86 cm−1, which indicates a strong interaction of TOPO with D2EHPA through this functional group. Significant changes during the transition of D2EHPA dimers to the monomeric form are also noticeable by the shift of the P-O-H bond signal from 1012.63 to 987.55 cm−1.
To confirm the formation mechanism, the 31P spectrum of HDES D2EHPA:TOPO 8:2 was also recorded (Figure S2). The characteristic signals of phosphorus atoms for both substances have a significant shift in different directions, which confirms the redistribution of the electron density towards the P=O double bond in TOPO. At the same time, for the signal of the phosphorus atom in D2EHPA, the shift intensity is less, which is probably due to the opposite effects, the transition of D2EHPA dimers to the monomeric form and the participation of a proton in a hydrogen bond with the TOPO molecule.
Density, which affects the separation of the aqueous phase and the HDES phase, and viscosity, which affects the mass transfer rate in the extraction system, are important properties of the synthesized HDES. Under the conditions of the extraction experiment, the density of HDES D2EHPA:TOPO 8:2 is 0.9475 g/cm3 (Figure 1a), and the viscosity is 88 mPa·s (Figure 1b), which is less than the density (0.97 g/cm3) and viscosity (180 mPa·s) of pure D2EHPA. The refractive index is also an important parameter characterizing the electronic polarizability of a molecule (Figure 1c). Its significance is important for understanding intermolecular interactions in a mixture and for establishing possible applications and behavior of an extractant in industrial and chemical processes. As the temperature increases, the physical properties (Figure 1a–c) of the studied HDES decrease, which correlates with the literature data [11].
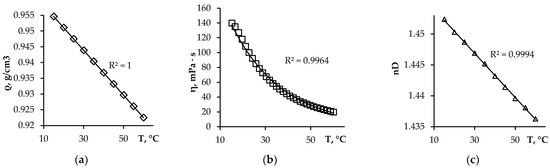
Figure 1.
Dependence of (a) the density, (b) the dynamic viscosity, and (c) the refractive index of HDES D2EHPA:TOPO on the temperature.
Another important characteristic of HDES formation is the solid–liquid state diagram. Figure 2 shows the HDES D2EHPA:TOPO phase diagram, which clearly shows the eutectic nature of the mixture. The decrease in temperature at the eutectic point (D2EHPA:TOPO 8:2) relative to pure D2EHPA was about 52 °C (Teut = −100.1 °C), which is a rather strong decrease in temperature and indicates imperfect mixing in this mixture.
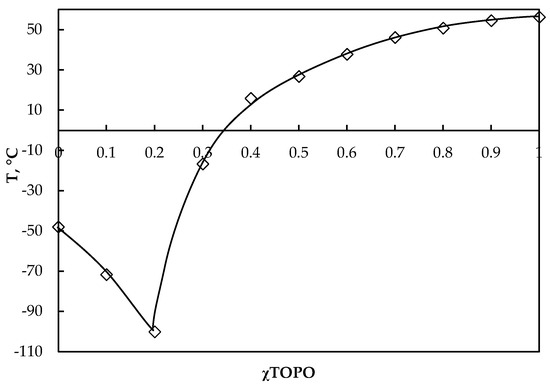
Figure 2.
Measured solid–liquid equilibria HDES D2EHPA:TOPO.
3.2. Extraction of Co(II) u Al(III) with D2EHPA:TOPO HDES
The time to reach thermodynamic equilibrium is one of the key parameters of the extraction process. In Figure 3a, the kinetics data for the extraction of metal ions in the HDES–water system in the range from 1 to 90 min is shown. It can be seen from the graphs obtained that the achievement of thermodynamic equilibrium in the case of Co ions occurs much faster (5 min.) than for Al ions (60 min.), which is presumably due to their charge and, as a consequence, steric hindrance in the case of a triply charged Al ion.
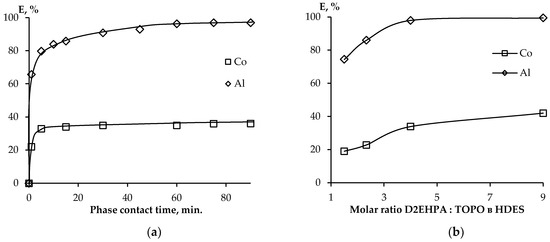
Figure 3.
Dependence of the degree of Co(II) and Al(III) extraction on (a) the phase contact time and (b) the ratio of D2EHPA:TOPO in HDES.
The influence of the ratio of components in HDES on the extraction efficiency was also studied. From Figure 3b, it can be seen that the extraction efficiency increases with an increase in the proportion of D2EHPA in HDES, which indicates a preferential cation exchange extraction of metal ions by D2EHPA.
Another important parameter for varying the extraction efficiency is the pH value of the initial aqueous phase. Figure 4a shows the dependences of the influence of pH of the aqueous phase on the degree of recovery of Co(II) and Al(III) using HDES D2EHPA:TOPO in the pH range 0–7. According to the data obtained, it can be seen that with increasing pH, the degree of extraction increases. With an increase in the pH value from 0.7 to 2.5 for Al(III), a sharp increase in the extraction indices is observed, and at pH > 2.5, its almost quantitative extraction into the HDES phase is achieved. In the case of Co(II), there is also an increase in the degree of extraction, however, the maximum values are reached at pH 5–35%. The nature of this dependence also indicates a cation exchange mechanism of extraction, which confirms that D2EHPA is the predominant extractant in this HDES. Thus, the extraction of Co(II) and Al(III) ions can be represented in general form by the following equation:
Men+(aq) + nHR(HDES) ↔ MeRn(HDES) + nH+(aq)
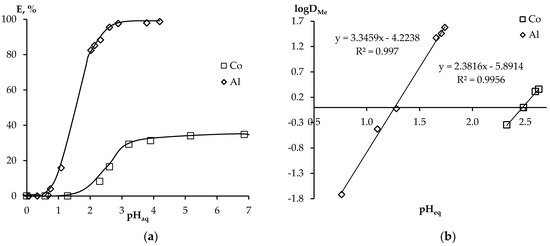
Figure 4.
Dependence of the degree of Co(II) and Al(III) extraction on (a) the phase contact time and (b) the ratio of D2EHPA:TOPO in HDES.
Also, the change in lgDMe, depending on the change in the pH of the equilibrium aqueous phase, should be linear with a slope of 2 for Co(II) and 3 for Al(III) [12], which is confirmed by the results in Figure 4b. An analysis of the data obtained enables us to conclude that metal ions from nitrate solutions using HDES D2EHPA:TOPO are extracted in the form of the Men+ cation.
Thus, by varying the process conditions and the ratio of HDES components, it is possible to achieve the separation of Co and Al ions from their mixture in aqueous solutions.
4. Conclusions
In this work, for the first time, a promising hydrophobic DES based on D2EHPA and TOPO as an extractant for the separation of Co(II) and Al(III) ions in nitrate media was proposed. The formation of HDES D2EHPA-TOPO was confirmed using NMR and FT-IR spectroscopy. A solid–liquid state diagram was performed and a eutectic composition (D2EHPA:TOPO 8:2) was obtained, the temperature of which is lower than pure D2EHPA by 52 °C. Depending on the temperature, the density, viscosity, and refractive index were studied, the values of which are suitable for carrying out extraction processes. The kinetics of extraction, the influence of the pH of the aqueous phase, and the influence of the ratio of components in HDES on the extraction of Co(II) and Al(III) were studied. The nature of the dependencies obtained indicates the predominant cation-exchange mechanism of extraction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ECP2023-14656/s1, Figure S1: Infrared spectra of D2EHPA, HDES and TOPO, Figure S2: 31P NMR spectra of D2EHPA, HDES and TOPO.
Author Contributions
Conceptualization, A.V.K. and Y.A.Z.; methodology, A.V.K. and Y.A.Z.; software, A.V.K.; validation, A.A.V., I.V.Z., N.A.M., and Y.A.Z..; formal analysis, Y.A.Z.; investigation, A.V.K.; resources, Y.A.Z. and A.A.V.; data curation, Y.A.Z.; writing—original draft preparation, A.V.K.; writing—review and editing, Y.A.Z. and A.A.V.; visualization, A.V.K.; supervision, A.A.V.; project administration, A.A.V.; funding acquisition, A.A.V. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by a grant from the Russian Science Foundation (project No. 20-13-00387), https://rscf.ru/en/project/20-13-00387/ (accessed on 15 May 2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Or, T.; Gourley, S.W.D.; Kaliyappan, K.; Yu, A.; Chen, Z. Recycling of Mixed Cathode Lithium-ion Batteries for Electric Vehicles: Current Status and Future Outlook. Carbon Energy 2020, 2, 6–43. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Quest for Green-Solvent Design: From Hydrophilic to Hydrophobic (Deep) Eutectic Solvents. ChemSusChem 2019, 12, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Bian, X.; Han, W.; Cao, D.; Wen, Y.; Zhu, K.; Wang, S. The Application of Deep Eutectic Solvents in Lithium-Ion Battery Recycling: A Comprehensive Review. Resour. Conserv. Recycl. 2023, 188, 106690. [Google Scholar] [CrossRef]
- Kozhevnikova, A.V.; Zinov’eva, I.V.; Zakhodyaeva, Y.A.; Baranovskaya, V.B.; Voshkin, A.A. Application of Hydrophobic Deep Eutectic Solvents in Extraction of Metals from Real Solutions Obtained by Leaching Cathodes from End-of-Life Li-Ion Batteries. Processes 2022, 10, 2671. [Google Scholar] [CrossRef]
- Kozhevnikova, A.V.; Milevskii, N.A.; Zinov’eva, I.V.; Zakhodyaeva, Y.A.; Voshkin, A.A. A Flow-Chart for Processing of a Lithium-Manganese Battery Using HDES Aliquat 336/Menthol. Theor. Found. Chem. Eng. 2022, 56, 650–654. [Google Scholar] [CrossRef]
- Ferreira, D.A.; Prados, L.M.Z.; Majuste, D.; Mansur, M.B. Hydrometallurgical Separation of Aluminium, Cobalt, Copper and Lithium from Spent Li-Ion Batteries. J. Power Sources 2009, 187, 238–246. [Google Scholar] [CrossRef]
- Girgin, S.; Acarkan, N.; Sirkeci, A.A. The Uranium(VI) Extraction Mechanism of D2EHPA-TOPO from a Wet Process Phosphoric Acid. J. Radioanal. Nucl. Chem. 2002, 251, 263–271. [Google Scholar] [CrossRef]
- Vellaichamy, S.; Palanivelu, K. Preconcentration and Separation of Copper, Nickel and Zinc in Aqueous Samples by Flame Atomic Absorption Spectrometry after Column Solid-Phase Extraction onto MWCNTs Impregnated with D2EHPA-TOPO Mixture. J. Hazard. Mater. 2011, 185, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.R.; de Hasseth, J.A. Fourier Transform Infrared Spectroscopy; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Zheng, R.; Bao, S.; Zhang, Y.; Chen, B. Synthesis of Di-(2-Ethylhexyl) Phosphoric Acid (D2EHPA)-Tributyl Phosphate (TBP) Impregnated Resin and Application in Adsorption of Vanadium(IV). Minerals 2018, 8, 206. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Novel Benzilic Acid-Based Deep-Eutectic-Solvents: Preparation and Physicochemical Properties Determination. Fluid Phase Equilib. 2020, 522, 112752. [Google Scholar] [CrossRef]
- Zinov’eva, I.V.; Kozhevnikova, A.V.; Milevskii, N.A.; Zakhodyaeva, Y.A.; Voshkin, A.A. Extraction of Cu(II), Ni(II), and Al(III) with the Deep Eutectic Solvent D2EHPA/Menthol. Theor. Found. Chem. Eng. 2022, 56, 221–229. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).