Grindability, Energy Requirements and Gravity Separation of Quartz from Blast Furnace Ironmaking Slag by Shaking Table and Falcon Concentrator †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Initial Characterisation of Elemental and Mineralogical Composition of Ironmaking Slag and Basicity Ratios
3.2. Bond’s Work Index and Power Drawn Calculations for Ironmaking Slag
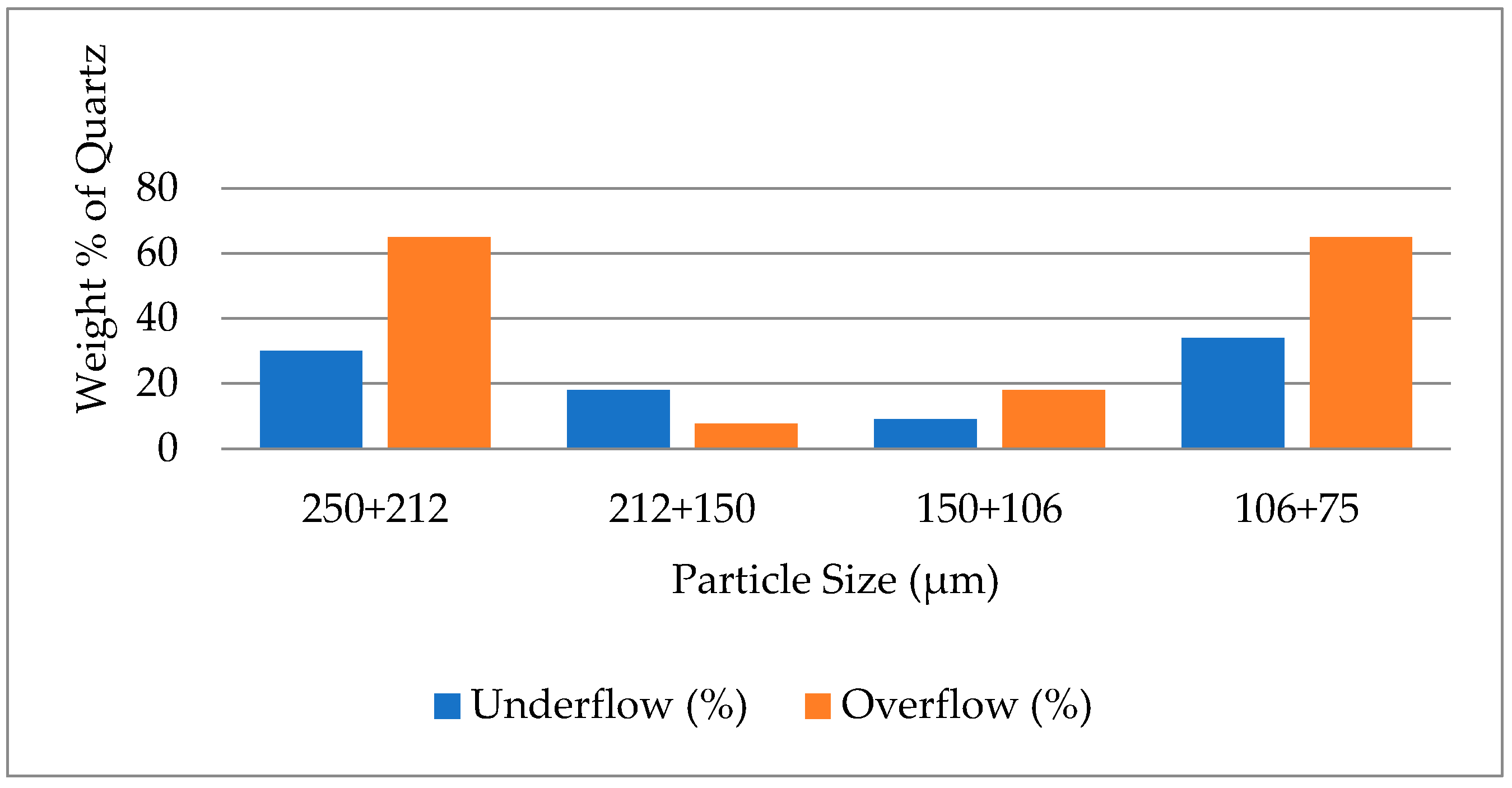
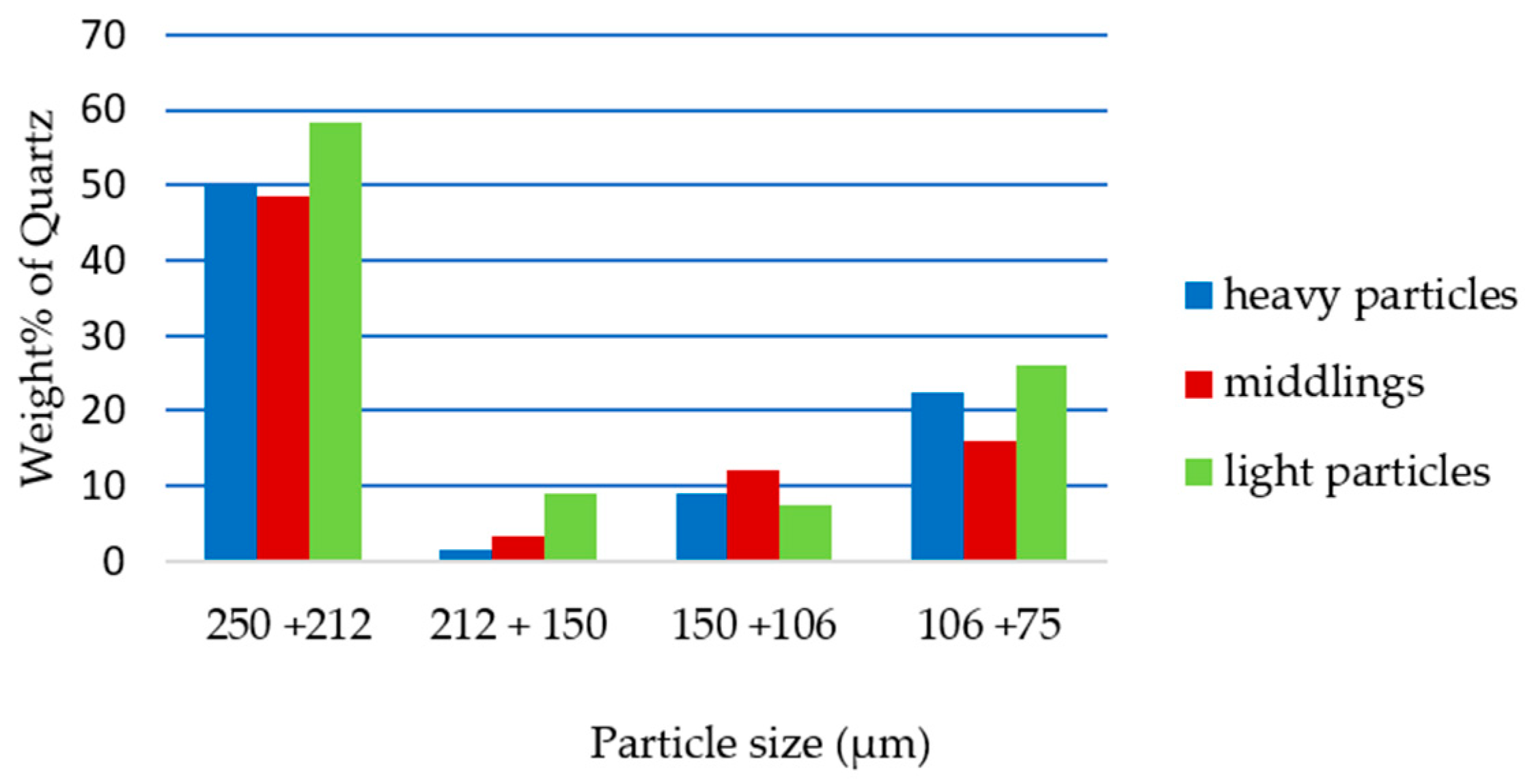
3.3. The Effect of Particle Size on Removal of Quartz from Ironmaking Slag by Falcon Concentrator
3.4. The Effect of Particle Size on Separation of Quartz from Ironmaking Slag by Shaking Table
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsukawa, K.; Shibata, T.; Kunitomo, K.; Ohno, K. A study on the utilization of iron and steel slag in civil engineering. Int. J. Geomate 2017, 12, 109–115. [Google Scholar]
- Ahmadi, R.; Shahsavari, A. Investigation of quartz flotation from decarburized vanadium bearing steel slag using shaking table and centrifugal concentrator. J. Clean. Prod. 2017, 161, 1085–1094. [Google Scholar]
- Deveau, C. Improving Fine Particle Gravity Recovery through Equipment Behavior Modification. In Proceedings of the 38th Annual Canadian Mineral Processors Conference, Ottawa, ON, Canada, 17–19 January 2006; pp. 501–517. [Google Scholar]
- Carrasco, C.; Quiroz, R.; Fuentes, P.; Henningsen, P. Comparison of grinding characteristics in high-pressure grinding roller (HPGR) and cone crusher (CC). Miner. Eng. 2016, 86, 105–114. [Google Scholar]
- Gupta, V.K.; Yan, D.S. Mineral Processing Design and Operations: An Introduction; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Chen, C.; Shi, F.; Xu, Z.; Wei, D. Performance of a multi-gravity separator for recovery of ultra-fine heavy minerals from coal fly ash. Miner. Eng. 2020, 156, 106539. [Google Scholar]
- Zhao, Y.; Huang, H.; Lu, H.; Liu, Z.; Lin, C. A review of microalgal biomass harvesting using flocculation. Bioresour. Technol. 2019, 280, 386–396. [Google Scholar]
- Wills, B.A.; Atkinson, K. An Introduction to Mineral Processing; Pergamon Press: Oxford, UK, 1993. [Google Scholar]
- Huitting, S.; Forssberg, E. An overview of recovery of metals from slag. Waste Manag. 2003, 23, 933–949. [Google Scholar]
- Huang, Y.; Guopong, X.; Huigao, C.; Junshi, W.; Yingfeng, W.; Hui, C. An overview of utilization of steel slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R. Influence of particle size on the carbonation of stainless-steel slag for CO2 storage. Energy Procedia 2009, 1, 4859–4866. [Google Scholar] [CrossRef]
- Cloete, M. Atlas on Geological Storage of CO2 in South Africa; Council for Geosciences: Pretoria, South Africa, 2010. [Google Scholar]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
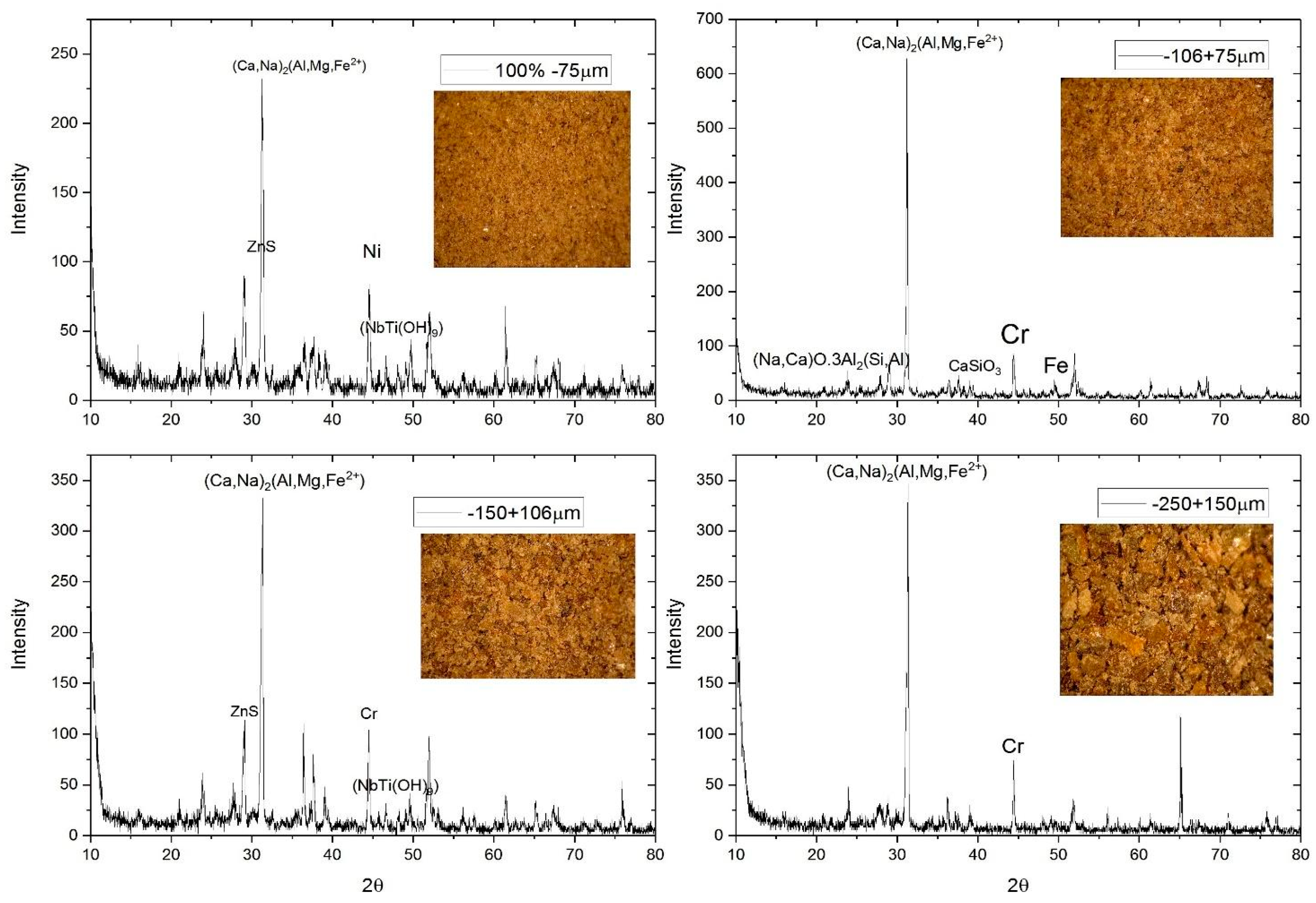
| Wt%SiO2 | Wt%CaO | Wt%Al2O3 | Wt%MgO | Wt% Fe2O3 | CaO/SiO2 | (CaO + MgO)/SiO2 |
|---|---|---|---|---|---|---|
| 38.46 | 32.84 | 13.85 | 8.63 | 1.77 | 0.85 | 1.08 |
| Grind Cycle | F80 | P80 | W at Wi = 24.9 | W at Wi = 13.8 | PDraw at Wi = 24.9 | PDraw at Wi = 13.8 |
|---|---|---|---|---|---|---|
| 1 | 2000 | 900 | 0.8050 | 0.9345 | 2.9453 | 2.7895 |
| 2 | 2000 | 1400 | 1.087 | 0.6020 | 3.2675 | 1.8094 |
| 3 | 2000 | 1000 | 2.306 | 1.2780 | 6.9264 | 3.8384 |
| 4 | 2000 | 400 | 6.882 | 3.8140 | 20.6554 | 11.4472 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohitlhetse, I.; Rutto, H.; Motsetse, K.; Manono, M. Grindability, Energy Requirements and Gravity Separation of Quartz from Blast Furnace Ironmaking Slag by Shaking Table and Falcon Concentrator. Eng. Proc. 2023, 37, 123. https://doi.org/10.3390/ECP2023-14691
Kohitlhetse I, Rutto H, Motsetse K, Manono M. Grindability, Energy Requirements and Gravity Separation of Quartz from Blast Furnace Ironmaking Slag by Shaking Table and Falcon Concentrator. Engineering Proceedings. 2023; 37(1):123. https://doi.org/10.3390/ECP2023-14691
Chicago/Turabian StyleKohitlhetse, Itumeleng, Hilary Rutto, Kentse Motsetse, and Malibongwe Manono. 2023. "Grindability, Energy Requirements and Gravity Separation of Quartz from Blast Furnace Ironmaking Slag by Shaking Table and Falcon Concentrator" Engineering Proceedings 37, no. 1: 123. https://doi.org/10.3390/ECP2023-14691
APA StyleKohitlhetse, I., Rutto, H., Motsetse, K., & Manono, M. (2023). Grindability, Energy Requirements and Gravity Separation of Quartz from Blast Furnace Ironmaking Slag by Shaking Table and Falcon Concentrator. Engineering Proceedings, 37(1), 123. https://doi.org/10.3390/ECP2023-14691







