Enhanced Removal of Cr (VI) from Wastewater with Green and Low-Cost Nanomaterials Using a Fuzzy Inference System (FIS) and an Artificial Neural Network (ANN) †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vincent, S.; Kandasubramanian, B. Cellulose nanocrystals from agricultural resources: Extraction and functionalisation. Eur. Polym. J. 2021, 160, 110789. [Google Scholar] [CrossRef]
- Kabuba, J.; Banza, M. Modification of clinoptilolite with dialkylphosphinic acid for the selective removal of cobalt (II) and nickel (II) from hydrometallurgical effluent. Can. J. Chem. Eng. 2021, 99, S168–S178. [Google Scholar] [CrossRef]
- Katha, P.S.; Ahmed, Z.; Alam, R.; Saha, B.; Acharjee, A.; Rahman, M.S. Efficiency analysis of eggshell and tea waste as Low cost adsorbents for Cr removal from wastewater sample. South African J. Chem. Eng. 2021, 37, 186–195. [Google Scholar] [CrossRef]
- Banza, M.; Rutto, H. Selective removal of Cr (VI) from hydrometallurgical effluent using modified cellulose nanocrystals (CNCs) with succinic anhydride and ethylenediaminetetraacetic acid: Isotherm, kinetics, and thermodynamic studies. Can. J. Chem. Eng. 2023, 101, 896–908. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mesbah, M.; Igwegbe, C.A.; Ezeliora, C.D.; Osagie, C.; Khan, N.A.; Dotto, G.L.; Salari, M.; Dehghani, M.H. Sono electro-chemical synthesis of LaFeO3 nanoparticles for the removal of fluoride: Optimization and modeling using RSM, ANN and GA tools. J. Environ. Chem. Eng. 2021, 9, 105320. [Google Scholar] [CrossRef]
- Banza, M.; Rutto, H.; Seodigeng, T. Soil and Sediment Contamination: An International Application of Artificial Neural Network and Shrinking Core Model for Copper (Ii) and Lead (Ii) Leaching from Contaminated Soil Using Ethylenediaminetetraacetic Acid Application of Artificial Neural N. Soil Sediment Contam. An Int. J. 2023, 1–21. [Google Scholar] [CrossRef]
- Husien, S.; El-taweel, R.M.; Salim, A.I.; Fahim, I.S.; Said, L.A.; Radwan, A.G. Review of activated carbon adsorbent material for textile dyes removal: Preparation, and modelling. Curr. Res. Green Sustain. Chem. 2022, 5, 100325. [Google Scholar] [CrossRef]
- Kabuba, J.; Banza, M. Results in Engineering Ion-exchange process for the removal of Ni (II) and Co (II) from wastewater using modi fi ed clinoptilolite: Modeling by response surface methodology and arti fi cial neural network. Results Eng. 2020, 8, 100189. [Google Scholar] [CrossRef]
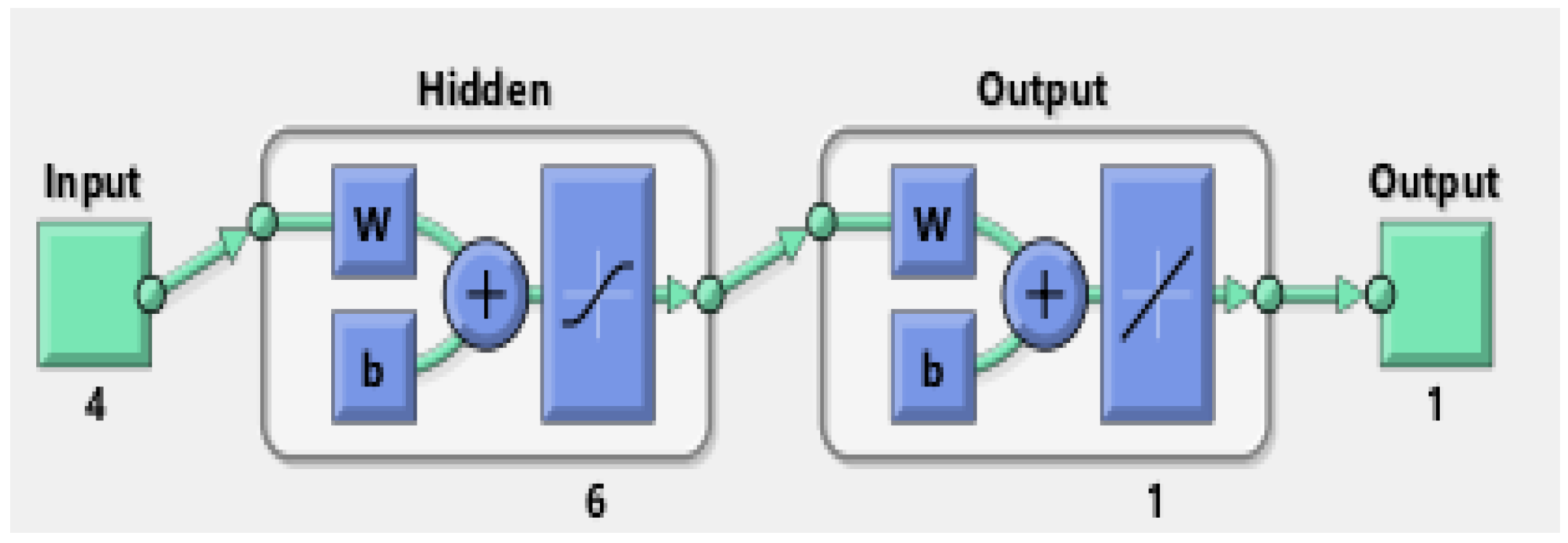

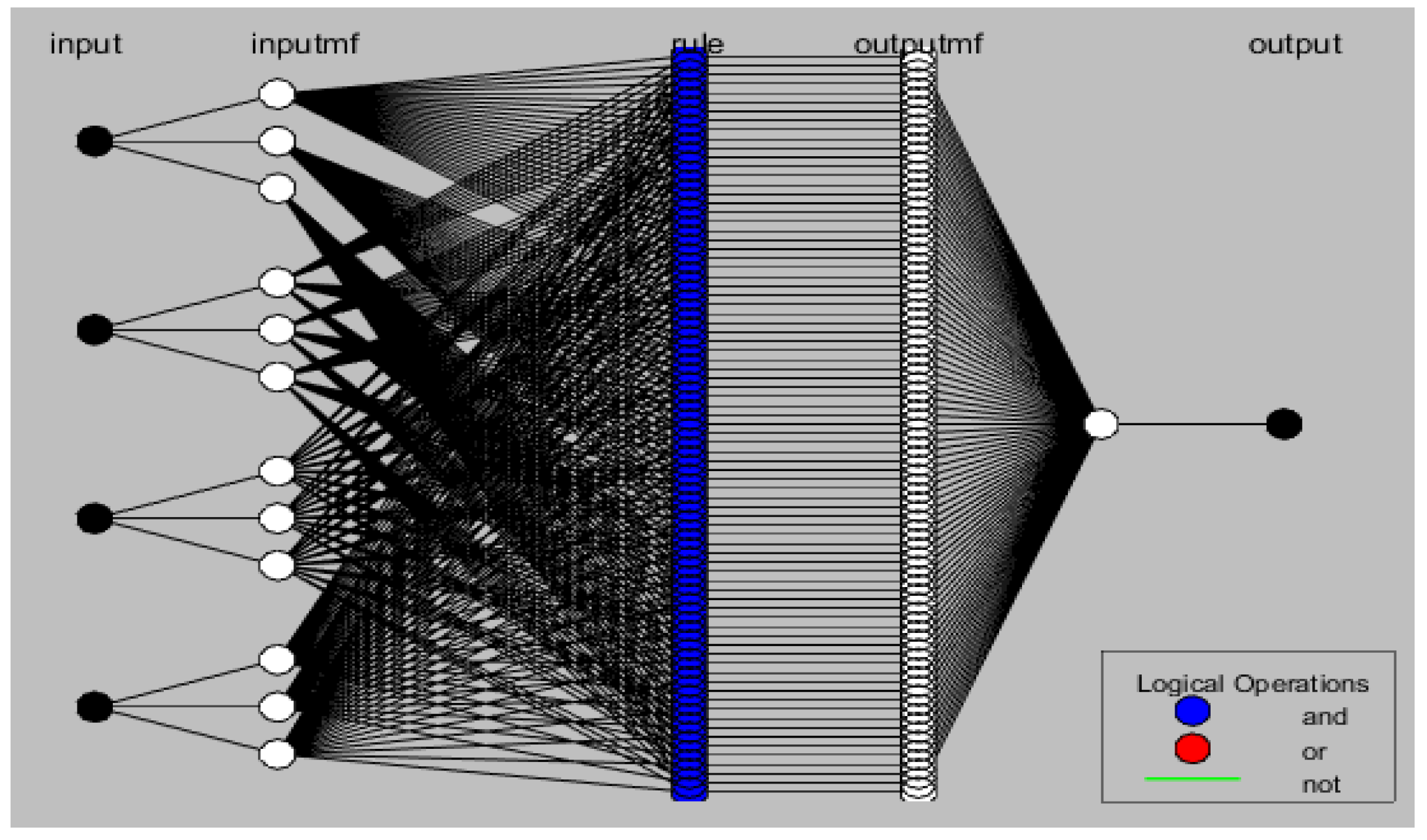

| Input | Range | Output |
|---|---|---|
| 1. pH | 3–9 | Removal percentage (%) |
| 2. Concentration Ni (II) | 50–500 | |
| 3. Time (min) | 0–120 | |
| 4. dosage adsorbent (mg/L) | 5–20 |
| pH | Conc (mg/L) | Time (min) | Dosage (mg/L) | Actual (%) | ANN (%) | ANFIS (%) |
|---|---|---|---|---|---|---|
| 6 | 250 | 120 | 10 | 92.00 | 92.2 | 92.7 |
| 6 | 250 | 60 | 10 | 93.01 | 92.7 | 93.07 |
| 3 | 50 | 0 | 20 | 79.52 | 78.05 | 78.9 |
| 9 | 50 | 120 | 5 | 82.94 | 84.82 | 83.89 |
| 3 | 500 | 0 | 20 | 75.51 | 75.28 | 76.14 |
| 9 | 50 | 0 | 20 | 72.47 | 71.90 | 71.12 |
| 6 | 250 | 60 | 10 | 93.92 | 93.24 | 93.82 |
| 6 | 250 | 60 | 10 | 93.97 | 93.51 | 93.77 |
| 9 | 50 | 120 | 20 | 76.70 | 76.28 | 77.27 |
| 3 | 50 | 0 | 5 | 81.54 | 81.25 | 81.54 |
| 6 | 50 | 60 | 10 | 84.92 | 84.85 | 84.73 |
| 3 | 500 | 0 | 5 | 84.08 | 83.25 | 83.79 |
| 9 | 500 | 0 | 20 | 71.05 | 71.02 | 71.08 |
| 9 | 500 | 120 | 20 | 75.05 | 74.56 | 74.28 |
| 6 | 250 | 60 | 10 | 93.91 | 93.24 | 92.71 |
| RMSE (Root means square errors) | 0.06 | 0.008 | ||||
| AARE (Absolute average relative errors) | 0.045 | 0.09 | ||||
| MSE (Mean square errors) | 0.035 | 0.035 | ||||
| R2 (Regression coefficient) | 0.98 | 0.99 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banza, M.; Seodigeng, T. Enhanced Removal of Cr (VI) from Wastewater with Green and Low-Cost Nanomaterials Using a Fuzzy Inference System (FIS) and an Artificial Neural Network (ANN). Eng. Proc. 2023, 37, 112. https://doi.org/10.3390/ECP2023-14677
Banza M, Seodigeng T. Enhanced Removal of Cr (VI) from Wastewater with Green and Low-Cost Nanomaterials Using a Fuzzy Inference System (FIS) and an Artificial Neural Network (ANN). Engineering Proceedings. 2023; 37(1):112. https://doi.org/10.3390/ECP2023-14677
Chicago/Turabian StyleBanza, Musamba, and Tumisang Seodigeng. 2023. "Enhanced Removal of Cr (VI) from Wastewater with Green and Low-Cost Nanomaterials Using a Fuzzy Inference System (FIS) and an Artificial Neural Network (ANN)" Engineering Proceedings 37, no. 1: 112. https://doi.org/10.3390/ECP2023-14677
APA StyleBanza, M., & Seodigeng, T. (2023). Enhanced Removal of Cr (VI) from Wastewater with Green and Low-Cost Nanomaterials Using a Fuzzy Inference System (FIS) and an Artificial Neural Network (ANN). Engineering Proceedings, 37(1), 112. https://doi.org/10.3390/ECP2023-14677





