Abstract
Acute toxicity data are a necessary component of the general analysis of gaseous environments and the prediction of the possible consequences of exposure to a chemical substance on living organisms. One of the fastest ways to obtain such information is to use gas-phase chemical sensors with sensitive layers of biological origin. Here we report an experimental study of complex loadings for classical quartz crystal microbalances arising in closely packed protein layers of ovalbumin (OVA) on the surface of polycrystalline silver, silver coated with rigid carbon fullerene C60, or a soft molecular-organic crystal of copper phthalocyanine (CuPc). OVA molecules are similarly immobilized on the silver and fullerene-decorated surfaces, while the response of the OVA-CuPc layer indicates an insignificant amount of protein on the surface. A systematic study of the kinetics of the responses of these layers to saturated vapors of volatile solvents shows that the QCM resonant frequency change correlates well with the toxicity of gaseous analytes. It has been observed that saturated vapors of water, ethanol, and their mixtures are classically adsorbed with a high adsorption capacity. Benzene and isobutanol showed only a non-monotonic anti-Sauerbrey behavior, while acetone and cyclohexane had a 10-fold smaller quasi-classical response. The possibility of a gaseous analyte changing not only the QCM loading but also the mechanical behavior of the protein mass associated with the surface opens up the possibility of observing nonspecific conformational changes in proteins, which can be the cause of general cytotoxicity. This effect, combined with the native conformation of OVA in densely packed protein films, allows the use of ovalbumin in creating sensitive bio-sniffer layers for fast toxicological assays—a new class of express tests for biosafety and environmental control.
1. Introduction
The new generation of advanced biochemical sensors implies the need to search for specific architectures of sensitive layers with predetermined selectivity. However, modern chemistry is still unable to predict and synthesize chemical structures with precisely tuned chemical functionality. This explains the fact that an increasing number of researchers are testing the molecular biosystems of living organisms with their predetermined functionality as sensitive layers of sensors [1,2].
Obviously, biomolecular machines are designed to operate in a liquid environment, so their capabilities are possible to realize only if their native conformation is preserved. At the same time, a number of protein molecules are characterized by an exceptionally high resistance of their spatial structure to drying. Egg white albumin (ovalbuminum, OVA) occupies a place of honor among such proteins. OVA has been a classic object of molecular biology since 1889, when it was one of the first proteins isolated in its pure form.
The soluble globular protein ovalbumin has a molecular mass of c.a. 43 kDa and is composed of 385 amino acids; approximately one-half of the amino acid residues are hydrophobic, and one-third of them are acidic and charged amino acids [3]. In the crystal structure, OVA presents itself as a slightly elongated ellipsoid with dimensions of 70 × 45 × 50 Å3 (an effective spherical diameter of 5 nm). In solution, OVA forms dimers without further aggregation due to the net negative charge of acidic residues on the protein surface [4]. The secondary structure of ovalbumin consists of 30% α-helix and 32% β-sheet structures (α-helices are relatively rigid, whereas β-strands are more flexible). OVA contains six cysteine residues, two of which are involved in a disulfide bond, while the rest four include free sulfhydryl; all of them are masked in the native state.
As with other albumins, OVA binds well to a wide variety of substances, including those that are poorly soluble in water; the adsorption capacity can reach more than 10% of the protein weight. Such features of albumins allow us to consider their coatings as promising sensitive layers in gaseous biosensors (so-called “bio-sniffers” [1,2,5,6]). In this work, we study the possibility for OVA layers deposited on different substrates (namely, silver, silver coated by buckminsterfullerene C60, and silver coated by copper phthalocyanine CuPc) to be a sensitive coating in QCM transducers to detect various analytes such as water, alcohols, acetone, cyclohexane, and benzene.
2. Materials and Methods
Solutions of lyophilized powder of ovalbumin (Sigma-Aldrich, Saint Louis, MO, USA) in bidistilled water were prepared immediately before applications. Piezoelectric resonators RK-169 with silver electrodes 400 nm thick and 8 mm in diameter were used as substrates. An amount of 7 µL of an OVA solution was applied on one side of the native resonator and one side covered by 100 nm films of CuPc or C60 (thermal deposition in vacuum) and then dried at room temperature for 24 h.
Biolayers were obtained by the environmentally controlled self-assembly of protein molecules, using the inherent tendency of proteins to predetermine their organization. Drying-mediated assembly is driven by the interplay of specific and non-specific interactions under conditions of volume reduction due to solvent evaporation. The gradual packing of proteins is based on both unspecific entropy effects and the surface functionality of biomolecules. Protein deposition on the surface of a physical transducer using this technology is well suited for center-symmetrical quartz crystal microbalance transducers with a central electrode diameter of 5–10 mm. As is known, the sensitivity of such transducers has a domed shape with a maximum in the center of the electrode and drops to almost zero at its edge [7]. The deposition of a protein droplet with a size slightly larger than the electrode diameter allows (1) to optimally implement the process of self-assembly of a homogeneous protein layer on the substrate surface and (2) to remove “unused” protein residues beyond the QCM sensitivity region. Quite a lot of literature is devoted to the mechanism of this seemingly simple process, since this approach has been widely used in medical diagnostics for more than a century [8]. The most important aspect of this approach for this work is that, in the presence of protein in solution, the evaporation front is formed in the direction from the center to the periphery, as a result of which only the adsorbate layer remains in the center and all excess protein is removed to the periphery. Since ovalbumin is a globular protein incapable of forming multilayer coatings, it is only necessary to ensure the initial formation of a monolayer of native protein molecules on the surface. For metal surfaces of gold or silver, this can be achieved by simply increasing the concentration of albumin in solution (in the volume of a drop) to more than 1 mg/mL [9]. In this case, the competition between the process of protein adsorption in its native form and the process of protein unfolding induced by the surface [10] is strongly shifted towards adsorption. The resulting dense adsorbate layer spatially blocks potential processes of loss of the tertiary structure; protein stabilization is based on dampening the molecular motions and therefore eliminating conformational transitions while the protein is still in the native state. This method is widely used, for example, to maintain the native form of glucose oxidase in glucose strip tests. Ultimately, the combination of (1) protein droplet drying characteristics, (2) spatial distribution of QCM sensitivity and high protein concentration in solution, and (4) ovalbumin resistance to denaturation in the absence of mechanical damage (for example, during shaking) makes it possible, using a simple technological procedure, to obtain homogeneous native ovalbumin monolayers on the surface of QCM transducers, including bio-sniffers. Despite the ability to bind hydrophobic analytes, albumin molecules typically do not demonstrate strong fixation on the surface of typically hydrophobic organic molecular crystals; the sensor responses were comparable regardless of whether or not the protein was applied to the surface [2]. So, on the CuPc surface, the island character of the coating is more likely in the places of the defects, with a small total number of protein molecules in other areas.
The original multichannel QCM analyzer was used for analysis. The instrument contains (i) a temperature-stabilized (21 ± 1 °C) chamber with the 8-sensor array; (ii) quartz generators (10 MHz); (iii) the microprocessor-based frequency meter (AT89C2051); (iv) a generator of the gas mixtures with argon as carrier gas (flow rate c.a. 180 mL/min, dynamic headspace injection through surface evaporation vapor generation [11]); and (v) a data-processing package [12,13]. The measurement procedure includes argon circulation up to the frequency stabilization (3 Hz); circulation of the vapor-argon mixture; purging by carrier gas up to the restoration of the initial frequency value of the QCMs.
QCM kinetics were analyzed with a model that takes heterogeneous processes on the surface into account using a stretched exponential function [14,15,16]:
where Rsat is the saturation level of the response, τ is the characteristic time constant, and β is the parameter that indicates the mechanism of surface layer evolution.
3. Results and Discussion
It has often been suggested that the air–water interface is a hostile environment for proteins [17]. For bio-sniffers, denaturation of biomolecules (when rigid globular proteins unfold into labile, expanding random coil chains with increased intrinsic viscosity) may happen at any stage of sensing layer preparation or under exploitation. However, for OVA, lyophilization typically leads to the native conformation of the protein. Moreover, close-packed proteins in a monolayer are spatially restricted for significant conformational changes [9]. We assume a native conformation of OVA in the film.
Native proteins have an extremely high packing density; the average packing density (c.a. 0.75) is slightly higher than that of spheres (c.a. 0.74). It is commonly accepted that non-polar residues of soluble globular proteins seclude themselves in a hydrophobic interior core that is shielded from the surrounding environment by charged, polar hydrophilic residues on the surface. The overall conformation is mainly governed by disulfide linkages along with cross-links such as hydrogen bonds (α-helix and β-strands, etc.) and hydrophobic interactions (within the core area). The addition of organic solvent can alter the conformational flexibility, leading to an overall change in the structure of the protein molecule.
Much controversy still surrounds the underlying mechanism of the action of organic solvents on proteins. Thus, polar and hydrophilic solvents such as ethanol and acetone have been found to stabilize (inhibit denaturation) protein structure at low concentrations, although the same solvents denature proteins at high concentrations. On the other hand, polar and hydrophobic toluene or chloroform showed only a destabilizing effect (increased rate of denaturation), while non-polar and hydrophobic pentane had no effect over a wide range of concentrations [18]. It has been suggested that chemical denaturants may alter the native structure by disrupting the hydrophobic core of proteins [19].
3.1. Water, Ethanol, and Mixture of Them
Typical adsorption curves for QCM sensors in a flow of saturated vapors of water and ethanol are shown in Figure 1; the adsorption is characterized by a monotonous decrease of the QCM resonant frequency, which is typical for the classical Sauerbrey model [20]. It means that there are no significant changes in the mechanical properties of both the protein itself and protein-surface contacts; protein molecules retain their conformation and are rigidly bound to the surface. A similar picture is observed in water-alcohol mixtures, such as, for example, brandy (Figure 1c). The low response of the sensor with the copper phthalocyanine sublayer, as noted above, is probably due to the small amount of protein bound to the QCM transducer.
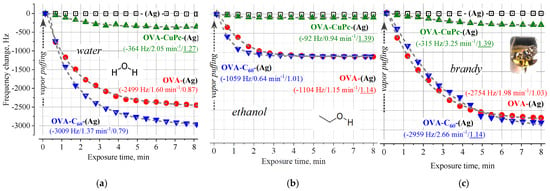
Figure 1.
The dependences of the response of QCM transducers modified with OVA overlayers when saturated vapor of water (a), ethanol (b), or brandy (c) is pumped over their surface. The approximation of the responses by stretched exponential functions is shown by a dashed line; the parameters of the best fit are shown in parentheses next to the corresponding curve and highlighted in color (Rsat, τ, β).
The adsorption capacities for both analytes significantly exceed the values typical for conventional organic coatings [12,13,14], which is well explained by the developed surface of the protein layer and the presence of a large number of groups suitable for binding. Approximation of the responses by stretched exponential functions [14,15,16] shows that Sauerbrey’s mass loading is due to Langmuir adsorption for ethanol (β~1) (Figure 1b) and a small contribution of the sorption for water (β~0.8) (Figure 1a). An order of magnitude lower adsorption capacity (taking into account a threefold difference in molecular weights), β = 1, and shorter characteristic times indicate that ethanol molecules do not penetrate inside the OVA layer. The surface analyte binding feature and fixation of the protein in a closely packed film manner prevent the denaturation of OVA under exposure to ethanol vapor; this is consistent with the stabilization effect of ovalbumin in solutions with small ethanol additions [18].
Comparable values of τ and β (Figure 1) indicate that the OVA is immobilized in a similar way on metal (Ag) and structured carbon (fullerene C60) surfaces. In line with molecular dynamics simulations [21] and experiments [22], protein fixation is caused mainly by hydrogen bond hydrophilic interaction and van der Waals forces. The complex interplay between these terms makes heteroaromatic tryptophan (Trp), strongly conjugated arginine (Arg), and methylmecapto-containing methionine (Met) the residues with the strongest interactions. The binding of Trp is characterized by π_π interactions between the aromatic ring in Trp and the fullerene cage; these interactions may be sandwich-like or T-shaped. Interactions of the π_π type also govern the binding between Arg and C60 due to the conjugated guanidinium group in Arg. The Met interacts with C60 in virtue of a hydrophobic “hug” between its Met residue and the fullerene cage.
3.2. Acetone and Cyclohexane
In Figure 2, the responses of the same sensors are presented when they are exposed to a stream of saturated vapors of acetone and cyclohexane. In contrast to water and ethanol, the magnitude of the responses decreases by more than a factor of 10, the characteristic times decrease, and a weakly nonmonotonic behavior is observed after the initially classical adsorption process. The kinetics of classical adsorption (Figure 2) are characterized by very low beta values (c.a. 0.6) on the OVA-Ag coating, which indicate the occurrence of sub-diffusion to the substrate between protein molecules (Ag or C60) [14,15,16]. The decrease in response with time is probably due to the slippage between protein dimers inside the layer or the change in linking by which proteins are bound to the transducer surface after (or during) the adsorption process (Figure 2b) [23]. Acetone and cyclohexane can be physically adsorbed on the surfaces of hydrophobic C60 [24] and silver (the water contact angle is 104°) [25]. The presence of such an adsorbate in local cavities on the surface (Figure 2b) can lead to variations in the strength of the contact of the protein with the surface and, consequently, cause an increase in the frequency of QCM [23].
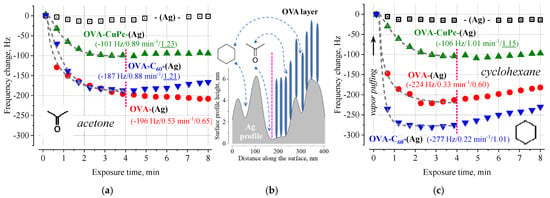
Figure 2.
Dependences of the response of QCM transducers modified with OVA overlayers when saturated vapors of acetone (a) and cyclohexane (c) are pumped over their surfaces. The approximation of the responses by stretched exponential functions is shown by a dashed line; the parameters of the best fit are shown in parentheses next to the corresponding curve and highlighted in color (Rsat, τ, β). The inset (b) shows an illustration of the silver surface profile (according to atomic force microscopy imaging [25]) and a layer of OVA dimers [4] at the same scale.
In general, the weak interaction of acetone and cyclohexane with OVA is not surprising since OVA is insoluble in those solvents and, moreover, acetone is widely used as a solution for precipitation and concentration of proteins. Both of these solvents cannot significantly affect the interior protein structure in a tightly packed globule because an acetone molecule may prefer to interact with the C60 surface and the protein interface. On the other hand, the non-polar cyclohexane molecule does not possess the capability to penetrate into the protein globule to give rise to the change in hydrophobic domain.
It is interesting to note the unusual behavior of the response for OVA-C60\OVA-(Ag) coating under the acetone/ethanol (Figure 1b, Figure 2a) exposure. The kinetic analysis shows that β > 1 in both cases, i.e., the adsorption process is self-accelerating [14,15,16]: when β < 1 processes at t < τ proceed faster than exponentially, whereas β > 1 acceleration takes place for processes when t > τ. A possible reason for that is some post-adsorption reorientation of the protein on the surface with the discovery of new unfilled areas. A similar effect was observed for all analytes in the OVA-CuPc layers. In this case, the adsorbate-induced protein’s “rolling” is not limited to surface coverage, which makes it possible to open previously hidden adsorption centers on the protein surface.
3.3. Benzene and Isobutanol
Radically different results were obtained with sensors exposed to a stream of benzene and isobutanol vapors. It should be noted that the isobutanol molecule possesses both hydrophilic (due to the HO-group) and hydrophobic (due to the isobutyl moiety) properties. In both cases (for benzene and isobutanol), a pronounced anti-Sauerbrey behavior is observed, which manifests itself after the initial short stage of classical adsorption (Figure 3).
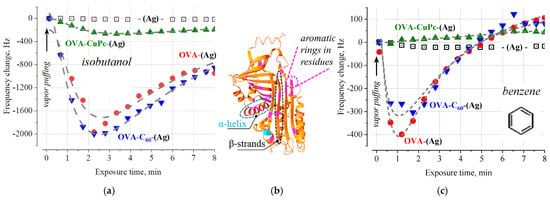
Figure 3.
The dependences of the response of QCM transducers modified with OVA overlayers when saturated vapors of isobutanol (a) and benzene (c) pumped over their surfaces. The dashed line is drawn only for the convenience of tracking the nature of the change in frequency. The inset (b) shows an illustration of the 3D structure of OVA (built according to PDB DOI: 10.2210/pdb1OVA/pdb using the graphic service of the RCSB Protein Data Bank); magenta highlights the position of 33 aromatic fragments of amino acid residues.
According to toxicological studies, both benzene and isobutyl alcohol denature proteins [18], while the molecular mechanisms of these processes are still poorly understood. It is assumed that under the sorption of the benzene molecule, its aromatic ring has the possibility of entering the globule, changing the flexibility of some protein structures [26].
Aromatic clusters between phenylalanine, tyrosine, and tryptophan residues (highlighted in color in Figure 3b) are very important for the stability of proteins but can be destroyed due to their interaction with benzene molecules.
It is well known that the effectiveness of alcohols as denaturing agents for proteins increases with increasing their hydrophobic chain length [27,28]. So, the effect of isobutanol can be interpreted in terms of hydrophobic interactions in the presence of HO-groups—the isobutanol molecules penetrate into the hydrophobic region of the protein and replace native hydrogen bonds, violating the predetermined native conformation.
A number of recent results confirm significant structural changes, finding that when OVA is subjected to denaturation conditions, it forms aggregates of various morphologies. In addition to the nanosheets with amyloid-like properties, flat ribbons were observed that were very different from the cylindrical forms of amyloid fibrils and relatively thick rod-like samples.
A short discussion of possible structures of denatured proteins caused by chemical denaturants allows us to assert the possibility of forming a wide variety of heterogeneous structures with sharply changing mechanical properties within the framework of a single biomolecule. The formation probability of certain structures critically depends on the nature of the specific agent causing the chemical denaturation of the native structure of proteins. According to [20], such structures with unevenly distributed masses and unequal mechanical interconnections are likely to be typical representatives of architectures in which the anti-Sauerbrey behavior of the QCM response is observed (Figure 3).
4. Conclusions
Several physical methods are available for the study of the structural changes accompanying the interaction of globular proteins with small molecules. The results obtained in this work allow us to conclude that bio-sniffers can also be used for the same purpose, in particular, for a qualitative assessment concerning the substance toxicity of proteins in vitro. Chemical denaturants destroy the internal bonds that hold the polypeptide chains together into tightly packed structures within the globule; the energy released in this case causes kinetic instability with subsequent internal rearrangements. Such approaches in the liquid phase are well known and are based on the fact that proteins determine the basic functions and vital activity of cells and the organism as a whole [29]. This approach is based on the idea that nonspecific conformational changes in proteins can be the cause of toxicity (the so-called hypothesis of general or basal cytotoxicity of B. Ekwall [30]). Our study allows us to propose a simple alternative approach to replace animal experiments in toxicological studies—methods that can be based on the assessment of impaired functions of proteins that are important for the body under the influence of toxicants. Multivariate sensor arrays [31,32] built on the basis of highly stable protein molecules (such as OVA, BSA, GST, GOD, etc.) can be used as the basis for expert systems for identification and monitoring highly toxic substances in the gas phase to control the atmosphere of a working area, enclosed spaces, or to solve environmental problems.
Finally, OVA molecules are similarly immobilized both on the surface of metals (silver) and structured carbon (fullerene C60); this is confirmed by the similar behavior of these coatings in reactions with gaseous analytes of various natures. The stability of OVA molecules in densely packed biofilms gives them the ability to create the bio-sniffer-sensitive layer for rapid toxicological tests in volatile vapors—a new class of rapid estimation for biosafety and environmental control.
Author Contributions
Conceptualization, B.S. and I.K.; methodology, B.S., I.K. and S.K.; validation, sample preparation S.K. and I.K.; investigation, I.K., S.K., J.B. and P.K.; writing—original draft preparation B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Snopok, B.; Kruglenko, I. Analyte induced water adsorbability in gas phase biosensors: The influence of ethinylestradiol on the water binding protein capacity. Analyst 2015, 140, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Kruglenko, I.; Burlachenko, J.; Grynko, D.; Belyaev, O. BSA films as sensitive coatings for gas sensors: Adsorption properties, application perspectives. Eur. Phys. J. Plus 2019, 134, 91. [Google Scholar] [CrossRef]
- Ting, B.C.P.; Pouliot, Y.; Gauthier, S.; Mine, Y. Fractionation of egg proteins and peptides for nutraceutical applications. In Separation, Extraction and Concentration Processes in the Food, Beverage and Nutraceutical Industries; Rizvi, S.S.H., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 595–618. [Google Scholar] [CrossRef]
- Ianeselli, L.; Zhang, F.; Skoda, M.W.A.; Jacobs, R.M.J.; Martin, R.A.; Callow, S.; Prévost, S.; Schreiber, F. Protein−Protein Interactions in Ovalbumin Solutions Studied by Small-Angle Scattering: Effect of Ionic Strength and the Chemical Nature of Cations. J. Phys. Chem. B 2010, 114, 3776–3783. [Google Scholar] [CrossRef]
- Arakawa, T.; Toma, K.; Mitsubayashi, K. Bio-Sniffer and Sniff-Cam; Elsevier: Amsterdam, The Netherlands, 2019; pp. 271–288. [Google Scholar] [CrossRef]
- Chien, P.-J.; Suzuki, T.; Tsujii, M.; Ye, M.; Toma, K.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. Bio-sniffer (gas-phase biosensor) with secondary alcohol dehydrogenase (S-ADH) for determination of isopropanol in exhaled air as a potential volatile biomarker. Biosens. Bioelectron. 2017, 91, 341–346. [Google Scholar] [CrossRef]
- Yakhno, T.; Yakhno, V. Structure and Dynamics of Aqueous Dispersions; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Snopok, B.; Laroussi, A.; Cafolla, C.; Voïtchovsky, K.; Snopok, T.; Mirsky, V.M. Gold surface cleaning by etching polishing: Optimization of polycrystalline film topography and surface functionality for biosensing. Surf. Interfaces 2020, 22, 100818. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Q.; Pan, W.; Yao, Y. Advances in the Mass Sensitivity Distribution of Quartz Crystal Microbalances: A Review. Sensors 2022, 22, 5112. [Google Scholar] [CrossRef] [PubMed]
- Snopok, B.; Kostyukevich, E. Kinetic studies of protein–surface interactions: A two-stage model of surface-induced protein transitionsin adsorbed biofilms. Anal. Biochem. 2006, 348, 222–231. [Google Scholar] [CrossRef]
- Burlachenko, J.; Kruglenko, I.; Snopok, B.; Persaud, K. Sample handling for electronic nose technology: State of the art and future trends. TrAC Trends Anal. Chem. 2016, 82, 222–236. [Google Scholar] [CrossRef]
- Kruglenko, I.V.; Snopok, B.A. Thin-film coating of dibenzotetraazaaannulenes for quantitative determination of hydro-chloric acid vapor by quartz crystal microbalance method. Theor. Exp. Chem. 2018, 54, 53. [Google Scholar] [CrossRef]
- Kruglenko, I.V.; Snopok, B.A.; Shirshov, Y.M.; Rowell, F.J. Multisensor systems for gas analysis: Optimization of the array for the classification of the pharmaceutical products. Semicond. Phys. Quantum Electr. Optoelectron. 2004, 7, 207–216. [Google Scholar] [CrossRef]
- Snopok, B.A.; Kruglenko, I.V. Nonexponential relaxations in sensor arrays: Forecasting strategy for electronic nose perfor-mance. Sens. Actuators B Chem. 2005, 106, 101–113. [Google Scholar] [CrossRef]
- Snopok, B.A. Nonexponential Kinetics of Surface Chemical Reactions (Review). Theor. Exp. Chem. 2014, 50, 67–95. [Google Scholar] [CrossRef]
- Snopok, B.A.; Snopok, O.B. Information Processing in Chemical Sensing: Unified Evolution Coding by Stretched Exponen-tial. In Nanostructured Materials for the Detection of CBRN; Springer: Berlin/Heidelberg, Germany, 2018; pp. 233–244. ISBN 978-94-024-1303-8. [Google Scholar]
- D’Imprima, E.; Floris, D.; Joppe, M.; Sánchez, R.; Grininger, M.; Kühlbrandt, W. Protein denaturation at the air-water interface and how to prevent it. Elife 2019, 8, e42747. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Adachi, K.; Schwartz, E. Stabilizing effect of various organic solvents on protein. J. Biol. Chem. 1978, 253, 6423–6425. [Google Scholar] [CrossRef]
- England, J.L.; Haran, G. Role of Solvation Effects in Protein Denaturation: From Thermodynamics to Single Molecules and Back. Annu. Rev. Phys. Chem. 2011, 62, 257–277. [Google Scholar] [CrossRef]
- Kravchenko, S.; Snopok, B. “Vanishing mass” in the Sauerbrey world: Quartz Crystal Microbalance study of self-assembled monolayers based on tripod-branched structure with tuneable molecular flexibility. Analyst 2020, 145, 656. [Google Scholar] [CrossRef]
- Marforio, T.D.; Calza, A.; Mattioli, E.J.; Zerbetto, F.; Calvaresi, M. Dissecting the Supramolecular Dispersion of Fullerenes by Proteins/Peptides: Amino Acid Ranking and Driving Forces for Binding to C 60. Int. J. Mol. Sci. 2021, 22, 11567. [Google Scholar] [CrossRef]
- Bingshe, X.; Xuguang, L.; Xiaoqin, Y.; Jinli, Q.; Weijun, J. Studies on the Interaction of Water-Soluble Fullerols with BSA and the Effects of Metallic Ions. MRS Proc. 2001, 675, 741. [Google Scholar] [CrossRef]
- Kruglenko, I.; Kravchenko, S.; Kruglenko, P.; Burlachenko, J.; Krishchenko, I.; Manoilov, E.; Snopok, B. Advanced Quartz Microbalance Sensors for Gas-Phase Applications: Effect of Adsorbate on Shear Bond Stiffness between Physical Transducer and Superlattice of Latex Nanoparticles. Eng. Proc. 2022, 27, 40. [Google Scholar] [CrossRef]
- Lundin, J.G.; Giles, S.L.; Cozzens, R.F.; Wynne, J.H. Self-Cleaning Photocatalytic Polyurethane Coatings Containing Modified C60 Fullerene Additives. Coatings 2014, 4, 614–629. [Google Scholar] [CrossRef]
- Kravchenko, S.A.; Kruglenko, I.V.; Snopok, B.A. Effect of the topography of silver films on the structure of surface ensembles of 11-mercaptoundecanol. Theor. Exp. Chem. 2009, 45, 108–113. [Google Scholar] [CrossRef]
- Herskovits, T.T.; Jaillet, H.; DeSena, A.T. On the Structural Stability and Solvent Denaturation of Proteins. J. Biol. Chem. 1970, 245, 6511–6517. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K. Kinetics of the Denaturation of Ovalbumin and S-Ovalbumin by Alcohols. Bull. Chem. Soc. Jpn. 1988, 61, 3043–3047. [Google Scholar] [CrossRef]
- Tufail, S.; Sherwani, M.A.; Shoaib, S.; Azmi, S.; Owais, M.; Islam, N. Ovalbumin self-assembles into amyloid nanosheets that elicit immune responses and facilitate sustained drug release. J. Biol. Chem. 2018, 293, 11310–11324. [Google Scholar] [CrossRef]
- Ekwall, B. The basal cytotoxicity concept. Alternative Melhods in Toxicology and the Life Sciences. In The World Congress on Alternalives and Animal Use in the Life Sciences: Education, Research, Testing; Goldberg, A.M., van Zupthen, L.F.M., Eds.; Mary Ann Liebert: New York, NY, USA, 1994; pp. 721–725. [Google Scholar]
- Burlachenko, Y.V.; Snopok, B.A.; Capone, S.; Siciliano, P. Performance of Machine Olfaction: Effect of Uniqueness of the Initial Data and Information Coding on the Discrimination Ability of Multisensor Arrays. IEEE Sens. J. 2011, 11, 649–656. [Google Scholar] [CrossRef]
- Burlachenko, J.V.; Snopok, B.A. Multisensor arrays for gas analysis based on photosensitive organic materials: An increase in the discrimination capacity under selective illumination conditions. J. Anal. Chem. 2008, 63, 610–619. [Google Scholar] [CrossRef]
- Snopok, B.; Kruglenko, I. Multisensor systems for chemical analysis: State-of-the-art in Electronic Nose technology and new trends in machine olfaction. Thin Solid Films 2002, 418, 21–41. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



