Abstract
Textile wastewaters are very problematic if released into the environment without proper treatment. Due to their recalcitrant nature, chemical treatments such as Advanced Oxidation Processes are often used. Among AOPs, Fenton-based processes presented promising results. However, considering the drawbacks associated with homogeneous Fenton, the aim of this work was to develop a heterogeneous catalyst using montmorillonite clay as the base material. After the application of an impregnation method, the Fourier-transform infrared spectroscopy (FTIR) and X-ray diffraction analysis showed the successful impregnation of iron into the montmorillonite clay. The catalyst Fe(II)-Mt was tested under different settings, and results showed that the best operational conditions (pH = 3.0, [Fe(II)-Mt 0.5M] = 1.0 g/L, [H2O2] = 4 mM, [MB] = 0.16 mM, radiation = UV-C (254 nm), time = 25 min) allowed to achieve 99.7% of Methylene blue (MB) removal. The catalyst showed great stability and was reused for three consecutive cycles. It can be concluded that Fe(II)-Mt catalyst is efficient for MB removal from aqueous solutions.
1. Introduction
Textile dyes, such as Methylene blue (MB) are used with frequency in operations of dying and printing of natural, synthetic, man-made, and mixed textile materials such as wool, silk, nylon, polyester, acrylic, polyacetate, and polyurethane [1]. Due to the textile dye wastewater (TDW) dark color, the sunlight is blocked, which in turn hinders the life of aquatic organisms. In addition, azo dyes such as MB constitute the biggest recalcitrant category of dyes on a commercial scale, which in turn represents a serious danger to the environment [2]. To treat these types of wastewater, advanced oxidation processes (AOPs) can be applied. The AOPs are based on the production of hydroxyl radicals () with an oxidation potential of 2.80 V. These radicals are very powerful, and react quickly and unselectively [3,4]. Among the AOPs, the Fenton-based processes were observed to be effective in the degradation of textile dyes [5], however, homogeneous Fenton has several issues associated, since is strongly affected by the (1) solution pH, which needs to be kept in the acidic range, (2) temperature of the reaction, (3) oxidant and catalyst concentrations, (4) neutralization of the solution after the reaction is complete. To avoid these drawbacks, heterogeneous Fenton-type catalysts can be developed, which include the incorporation of Fe ions or Fe oxides into porous supports [6]. The aim and major novelty of this work was the development of a new catalyst using montmorillonite clay as a base material, to degrade a textile dye.
2. Materials and Methods
2.1. Reagents
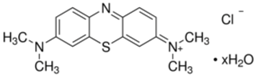
The activated sodium bentonite (Na-Mt) was supplied by Angelo Coimbra & Ca., Lda, Maia, Porto, Portugal, the iron(II) sulfate heptahydrate (FeSO4•7H2O) was acquired by Panreac, Castellar del Vallès, Barcelona, the hydrogen peroxide (H2O2 30% w/w) was acquired by Sigma-Aldrich, St. Louis, Missouri, EUA and the Methylene blue (MB) was acquired by VWR Chemicals, Llinars del Vallès, Barcelona, Spain. The molecular structure of MB in non-hydrolyzed form is illustrated in Table 1. NaOH and H2SO4 (95%) were both obtained from Analar Normapur. Deionized water was used to prepare the respective solutions. Deionized water was used to prepare the respective solutions.

Table 1.
Chemical structure, maximum absorbance, and molecular weight of MB [7].
2.2. Analytic Techniques
The maximum absorbance wavelength (λmax) of MB was found at 665 nm, and the concentration of the residual dye in solution was calculated by Beer–Lambert’s law (Equation (1)), using the optical density and molar extinction observed at the characteristic wavelength, as follows:
where A is the absorbency, l the path length (cm), ε the molar extinction coefficient (L/mol/cm) and C the dye concentration at time t (mol/L). Dye discoloration was analyzed by Equation (2), as follows [8,9]:
where Ct and C0 are the concentrations of dye at reaction time t and 0, respectively.
2.3. Catalyst Fe(II)-Mt Calcinated Clay Preparation
The Fe(II)-Mt calcinated clay was prepared by using the impregnation method [10]. A specific amount of FeSO4•7H2O was dissolved in a beaker containing distilled water. Then, the Na-Mt was added to this aqueous solution and was stirred constantly in a water bath at 100 °C until all water was evaporated. After impregnation, the sample was oven dried at 105 °C for 12 h, followed by calcination at 500 °C for 4 h in a muffle furnace. The structural characterization of the Na-Mt and Fe(II)-Mt catalyst was obtained from X-ray diffraction analysis (XRD) and Fourier-transform infrared spectroscopy (FTIR).
2.4. Heterogeneous Fenton-Based Experiments
The photocatalytic experiments were performed in a batch cylindrical photoreactor (500 cm3), equipped with a UV-C low-pressure mercury vapor lamp (TNN 15/32)—working power = 15 W (795.8 W m−2) and λmax = 254 nm (Heraeus, Hanau, Germany). After the reaction has started, 2 mL of dye solution was withdrawn at periodic intervals and analyzed in a UV–visible scanning spectrum 200–800 nm, using a Jasco V-530 UV-vis (Tokyo, Japan). The samples were filtrated by 0.20 µm filter and the Fe2+ concentrations were analyzed by atomic absorption spectroscopy (AAS) using a Thermo Scientific iCE 3000 SERIES, Waltham, Massachusetts, EUA. All the experiments were performed in triplicate and the observed standard deviation was always less than 5% of the reported values.
3. Results and Discussion
3.1. Characterization of Fe-BC Catalyst
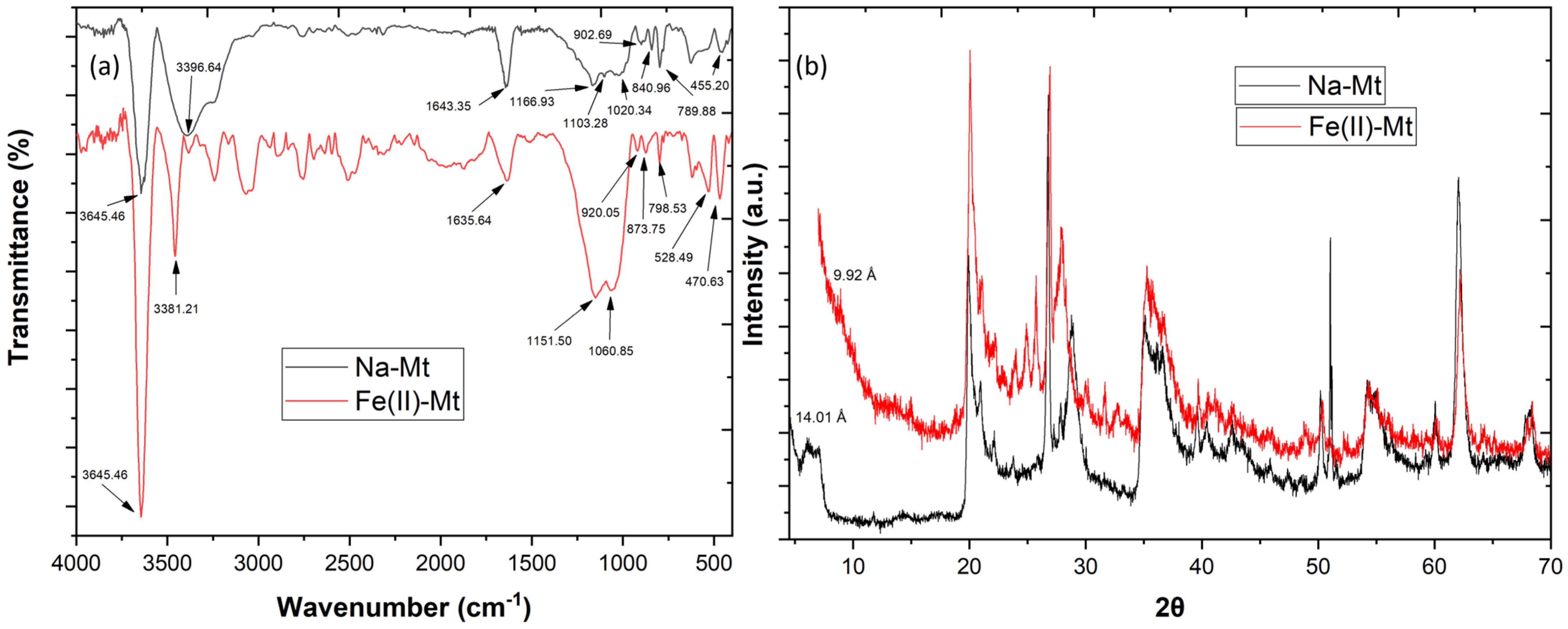
The FTIR analysis (Figure 1a) showed similar peaks between the Na-Mt and Fe(II)-Mt, such as 3645.46 cm−1 (structural O–H groups), 1643.35 cm−1 (adsorbed water yielded), 1103.28, 999.13 and 789.88 cm−1 (structural Si–O groups), 902.69 cm−1 (structural Al–Al–OH groups) and 883.40 cm−1 (structural Al–Fe–OH groups) [11,12]. These results mean that after calcination the montmorillonite clay kept its original structure. However, the Fe(II)-Mt revealed a significant structural change, with the disappearance of a peak at 1103.28 cm−1 and the appearance of a new peak at 528.49 cm−1.
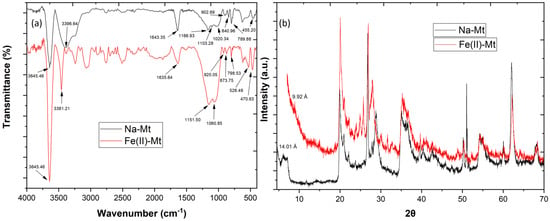
Figure 1.
Analysis of Na-Mt and Fe(II)-Mt by (a) FTIR and (b) X-ray diffraction.
The XRD patterns of both Na-Mt and catalyst Fe(II)-Mt are shown in Figure 1b, and the crystallographic parameters were evaluated by measuring the basal reflections in the plane dhkl 001. It was observed that Na-Mt exhibits a diffraction peak at the d001 plane at 2θ = 6.18° which corresponded to a basal spacing of 14.01 Å, a characteristic peak of montmorillonite clays. The data revealed a significant shift associated with the reflection d001, from 14.01 Å to 9.92 Å, confirming the structural modifications that occurred on the Fe(II)-Mt after the calcination.
3.2. MB Degradation by Heterogeneous Fenton-Based Processes
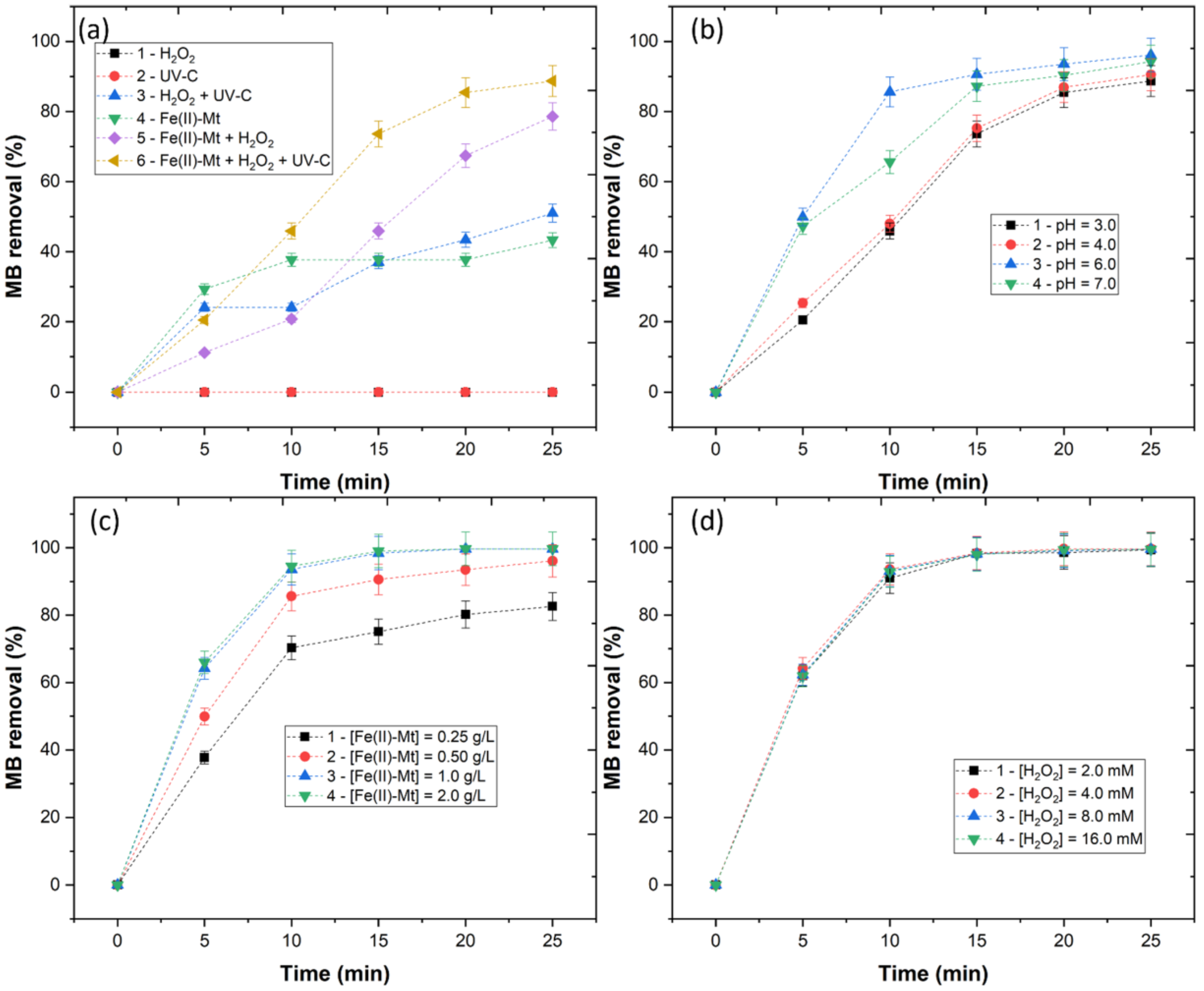
In the previous section, the successful impregnation of iron into the Na-Mt clay was shown. Before the application of the Fe(II)-Mt catalyst, it is necessary to understand the effect of the different AOPs in MB removal. In Figure 2a, six different processes were tested, with the following conditions: pH = 3.0, [Fe(II)-Mt 0.5M] = 0.5 g/L, [H2O2] = 4 mM, [MB] = 0.16 mM, radiation = UV-C (254 nm), time = 25 min. The application of UV-C and H2O2 had the lowest MB removal (<0.5%), since these processes were not capable of producing hydroxyl radicals (). With the combination of H2O2 + UV-C, MB removal achieved a removal of 51.0%, due to the direct photolysis of H2O2 by the UV radiation, which lead to the generation of radicals [13]. The application of Fe(II)-Mt reached 43.3% MB removal. This removal evidences the Fe(II)-Mt adsorption capacity, which is similar to works such as Flores et al. [14], who observed that heterogeneous catalysts have adsorption capacity. Finally, heterogeneous Fenton and photo-Fenton were applied, with results showing an MB removal of 78.6 and 88.7%. Clearly, the catalyst can convert the H2O2 and generate radicals. This effect is enhanced by the application of UV radiation, thus heterogeneous photo-Fenton was selected as the best AOP.
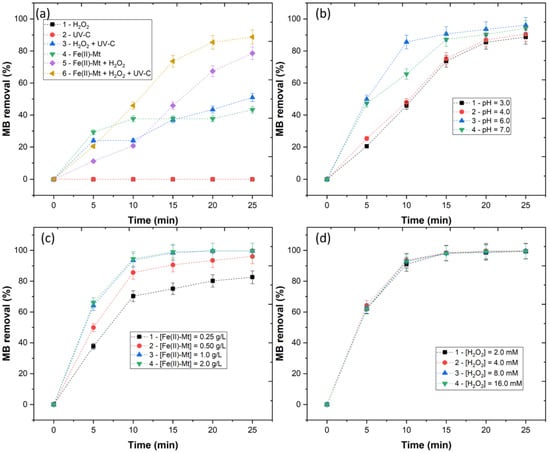
Figure 2.
Removal of MB by (a) variation of processes, (b) variation of pH (3.0–7.0) with [Fe(II)-Mt 0.5M] = 0.5 g/L, [H2O2] = 4 mM, [MB] = 0.16 mM, radiation = UV-C (254 nm), time = 25 min (c) variation of Fe(II)-Mt 0.5M catalyst concentration (0.25–2.0 g/L), with pH = 3.0, [H2O2] = 4 mM, [MB] = 0.16 mM, radiation = UV-C (254 nm), time = 25 min and (d) variation of H2O2 concentration (2.0–16.0 mM) with pH = 3.0, [Fe(II)-Mt 0.5M] = 1.0 g/L, [MB] = 0.16 mM, radiation = UV-C (254 nm), time = 25 min.
One of the major limitations regarding homogeneous Fenton is the precipitation of the catalyst at alkaline pH. Thus, the pH varied from 3.0 to 7.0 (Figure 2b). Results showed an MB removal of 88.7, 90.5, 96.1, and 94.2%, respectively, for pH 3.0, 4.0, 6.0, and 7.0. The increase in efficiency observed at pH 6.0 was similar to the work of Guimarães et al. [15], who applied pillared catalysts to treat winery wastewater. In addition, Portuguese legislation demands the final water pH to be between 6–9, and a further neutralization process is avoided, thus reducing additional costs.
The catalyst concentration was varied (0.25–2.0 g/L), to study its effect on MB removal. The results in Figure 2c showed an MB removal of 82.6, 96.1, 99.7, and 99.7%, respectively, for 0.25, 0.50, 1.0, and 2.0 g/L. As the catalyst concentration increased from 0.25 to 1.0 g/L, the production of radicals increased, due to a higher content of Fe2+ present in the solution. However, as the catalyst concentration increased further than 1.0 g/L, no significant changes were observed in MB removal. These results suggest that an excess of catalyst concentration may increase the solution turbidity hampering the UV radiation penetration and decreasing the MB removal efficiency.
Finally, the H2O2 concentration was varied from 2.0 to 16.0 mM to access the effect of the oxidant concentration in heterogeneous photo-Fenton (Figure 2d). The results showed an MB removal of 99.4, 99.7, 99.6, and 99.5%, respectively. The results showed that the removal of MB does not change for H2O2 concentrations higher than 2.0 mM. These results are in agreement with the work of Silva et al. [16], who observed that the concentration of H2O2 had no effect on the degradation of textile dyes by heterogeneous photo-Fenton.
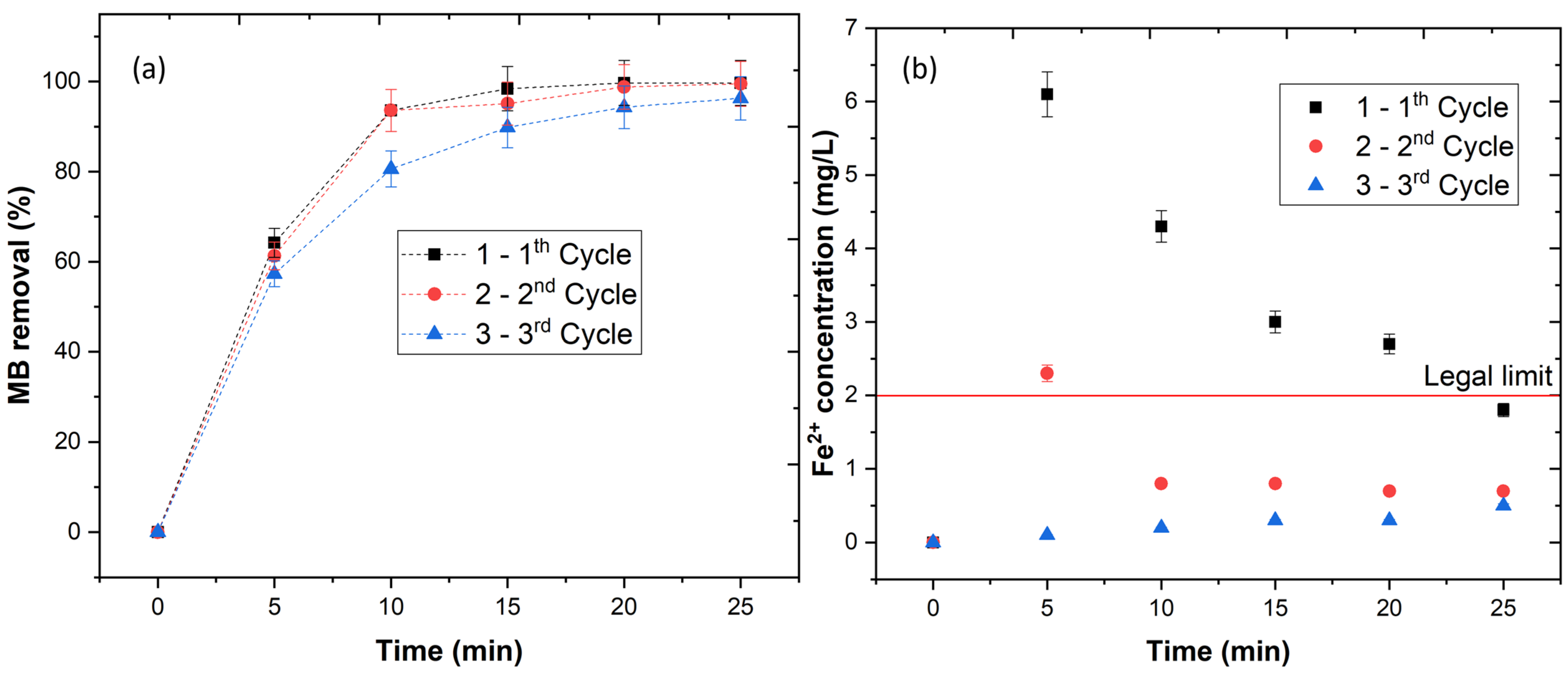
The results showed that Fe(II)-Mt catalyst is very efficient in the degradation of textile dyes, however, in order to reduce the operational costs in actual wastewater treatment plants (WWTP), the catalyst should be recovered and reused. Therefore, three consecutive cycles were performed. The results in Figure 3a showed an MB removal of 99.7, 99.5, and 96.3%, respectively, for the first, second and third cycles, respectively. These results showed that the catalyst releases the iron to the aqueous solution taking place the photo-Fenton process. Afterwards, the catalyst reabsorbs the iron at the end of the reaction and reuse it again for three cycles. In order to prove this idea, the leaching concentration was determined during the three cycles (Figure 3b). These results showed a high Fe2+ release during the first 5 min, decreasing its concentration from 5 to 25 min, clearly showing the absorption of iron back to the catalyst. In addition, the final Fe2+ concentration values were observed to be far below the legal limits for the discharge of treated waters—2 mg L−1 [16].
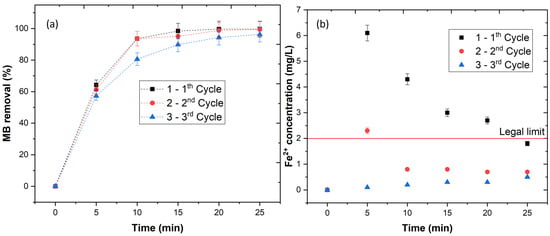
Figure 3.
(a) Catalyst stability, (b) Fe2+ leaching concentration for 3 consecutive cycles. Experimental conditions: pH = 3.0, [Fe(II)-Mt 0.5M] = 1.0 g/L, [H2O2] = 2.0 mM, [MB] = 0.16 mM, radiation = UV-C (254 nm), time = 25 min.
4. Conclusions
Based on the FTIR and X-ray diffraction analysis, it can be concluded that calcination of montmorillonite clays does not affect their structural integrity and allows the incorporation of Fe2+. Therefore, heterogeneous photo-Fenton is an efficient process for MB degradation and it is affected by the solution pH, concentration of catalyst and H2O2. Finally, the catalyst can be reused for, at least, three consecutive cycles, decreasing the treatment costs.
Author Contributions
Conceptualization, N.J. and A.R.T.; methodology, N.J.; software, N.J.; validation, N.J., M.S.L., and J.A.P.; formal analysis, N.J.; investigation, N.J.; resources, M.S.L. and J.A.P.; data curation, N.J.; writing—original draft preparation, N.J.; writing—review and editing, M.S.L. and J.A.P.; visualization, J.A.P.; supervision, M.S.L. and J.A.P.; project administration, J.A.P.; funding acquisition, J.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the financial support of the Project AgriFood XXI, operation n◦ NORTE-01-0145-FEDER-000041, and to the Fundação para a Ciência e a Tecnologia (FCT) for the financial support provided to CQVR through UIDB/00616/2020. Ana R. Teixeira also thanks the FCT for the financial support provided through the doctoral scholarship UI/BD/150,847/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El, A.; El, A. Textile Finishing Dyes and Their Impact on Aquatic Environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological Methods for Textile Dye Removal from Wastewater: A Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Jorge, N.; Santos, C.; Teixeira, A.R.; Marchão, L.; Tavares, P.B.; Lucas, M.S.; Peres, J.A. Treatment of Agro-Industrial Wastewaters by Coagulation-Flocculation-Decantation and Advanced Oxidation Processes—A Literature Review. Eng. Proc. 2022, 19, 33. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.R.; Lucas, M.S.; Peres, J.A. Combined Organic Coagulants and Photocatalytic Processes for Winery Wastewater Treatment. J. Environ. Manag. 2023, 326, 116819. [Google Scholar] [CrossRef]
- Tokumura, M.; Morito, R.; Hatayama, R.; Kawase, Y. Iron Redox Cycling in Hydroxyl Radical Generation during the Photo-Fenton Oxidative Degradation: Dynamic Change of Hydroxyl Radical Concentration Fe (II) Compounds Fe (III) Compounds. Appl. Catal. B Environ. 2011, 106, 565–576. [Google Scholar] [CrossRef]
- Herney-ramirez, J.; Silva, A.M.T.; Vicente, M.A.; Costa, C.A.; Madeira, L.M. Degradation of Acid Orange 7 Using a Saponite-Based Catalyst in Wet Hydrogen Peroxide Oxidation: Kinetic Study with the Fermi’s Equation. Appl. Catal. B Environ. 2011, 101, 197–205. [Google Scholar] [CrossRef]
- Paulino, T.R.S.; Araújo, R.d.S.; Salgado, B.C.B. Study of Advanced Oxidation of Basic Dyes by Fenton Reaction (Fe2+/H2O2). Eng. Sanit. e Ambient. 2015, 20, 347–352. [Google Scholar] [CrossRef]
- Teixeira, A.R.; Jorge, N.; Fernandes, J.R.; Lucas, M.S.; Peres, J.A. Textile Dye Removal by Acacia Dealbata Link. Pollen Adsorption Combined with UV-A/NTA/Fenton Process. Top. Catal. 2022, 1–17. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.R.; Matos, C.C.; Lucas, M.S.; Peres, J.A. Combination of Coagulation–Flocculation–Decantation and Ozonation Processes for Winery Wastewater Treatment. Int. J. Environ. Res. Public Health 2021, 18, 8882. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Hameed, B.H. Fe–Clay as Effective Heterogeneous Fenton Catalyst for the Decolorization of Reactive Blue 4. Chem. Eng. J. 2011, 171, 912–918. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.R.; Lucas, M.S.; Peres, J.A. Combination of Adsorption in Natural Clays and Photo-Catalytic Processes for Winery Wastewater Treatment. In Advances in Geoethics and Groundwater Management: Theory and Practice for a Sustainable Development; Abrunhosa, M., Chambel, A., Peppoloni, S., Chaminé, H.I., Eds.; Springer: Cham, Switzerland, 2021; pp. 291–294. ISBN 978-3-030-59320-9. [Google Scholar]
- Jorge, N.; Teixeira, A.R.; Guimarães, V.; Lucas, M.S.; Peres, J.A. Treatment of Winery Wastewater with a Combination of Adsorption and Thermocatalytic Processes. Processes 2022, 10, 75. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Marques, C.C.; Portugal, I.; Nunes, M.I. Fenton Processes for AOX Removal from a Kraft Pulp Bleaching Industrial Wastewater: Optimisation of Operating Conditions and Cost Assessment. J. Environ. Chem. Eng. 2020, 8, 104032. [Google Scholar] [CrossRef]
- Flores, Y.; Flores, R.; Gallegos, A.A. Heterogeneous Catalysis in the Fenton-Type System Reactive Black 5/H2O2. J. Mol. Catal. A Chem. 2008, 281, 184–191. [Google Scholar] [CrossRef]
- Guimarães, V.; Teixeira, A.R.; Lucas, M.S.; Silva, A.M.T.; Peres, J.A. Pillared Interlayered Natural Clays as Heterogeneous Photocatalysts for H2O2-Assisted Treatment of a Winery Wastewater. Sep. Purif. Technol. 2019, 228, 115768. [Google Scholar] [CrossRef]
- Silva, A.M.T.; Herney-ramirez, J.; Söylemez, U.; Madeira, L.M. A Lumped Kinetic Model Based on the Fermi’s Equation Applied to the Catalytic Wet Hydrogen Peroxide Oxidation of Acid Orange 7. Appl. Catal. B Environ. 2012, 121–122, 10–19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).