1. Introduction
Water is essential for the maintenance of human life. However, access to potable water is still rare for thousands of people. The environmental impacts of globalization and industrialization expansion are notable in several spheres, such as in aquatic ecosystems. One of the common ways of contamination of water resources is from industrial waste, namely through the discharge of organic dyes in water bodies. The use of these organic contaminants is increasing, and the lack of a correct treatment causes several environmental problems. Among these organic dyes are the azo group, where the compounds possess the -N=N- bond in their structure [
1]. All of these dyes are toxic, have a carcinogenic, mutagenic, and genotoxic potential, and can be very harmful to aquatic and human life [
2].
Therefore, it is important and necessary to search for new methodologies for the degradation of dyes and treatment of this wastewater. In recent years, the use of silver nanoparticles (AgNPs) has been highlighted for the nanocatalysis of these industrial dyes. These AgNPs arise with the expansion of nanotechnology and are the object of study in this research. Thus, the main objective of this work was to perform the catalytic degradation of the synthetic dyes Congo Red (CR) and Methyl Orange (MO) using of AgNPs, stabilized with polyvinyl alcohol (PVA) and polyvinylpyrrolidone (PVP).
2. Materials and Methods
2.1. Reagents
All solutions were prepared with ultrapure water, and no further purification was required. The reagents used in this work are listed below:
Silver Nitrate (AgNO3, 99%, Sigma-Aldrich, St. Louis, MO, USA)
Sodium Borohydride (NaBH4, 99.99% Sigma-Aldrich)
Sodium Citrate P.A. (Na3C6H5O7·2H2O, Dinamica, São Paulo, Brazil)
Polyvinylpyrrolidone (PVP, 95%, Dinamica)
Polyvinyl alcohol (PVA, 99%, Sigma-Aldrich)
Methyl Orange P.A. (C14H14N3NaO3S, Dinamica)
Congo Red (C32H22N6Na2O6S2, Dinamica)
2.2. Synthesis of AgNP-PVA and AgNP-PVP
For the synthesis of AgNPs, 0.1 mL of stabilizer (PVP and PVA) was added to 23,950 mL of water and shaken vigorously for 2 min. Then, 50 µL of silver nitrate (0.05 M) and 0.5 mL of sodium citrate (0.05 M) were added to the solution. Finally, 250 μL of sodium borohydride (100 mM) was added to this mixture to start the reduction process, changing its color immediately to light yellow. The reaction was kept under vigorous stirring and 50 °C for another 10 min.
2.3. Characterization of AgNPs
The AgNPs were characterized by UV-Vis absorption spectroscopy (Lambda 650 spectrophotometer, PerkinElmer, Waltham, MA, USA), transmission electron microscopy (FEI Tecnai Spirit Biotwin G2), surface zeta potential and dynamic light scattering (Zetasizer Nano ZS, Malvern Analytical, Malvern, UK), and Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-OES). The average size of the nanoparticles was determined using the ImageJ software with microscopy images.
2.4. Degradation of Congo Red and Methyl Orange Dyes
The study of the degradation of the dyes was carried out by UV-Vis absorption spectroscopy for up to 40 min, with intervals of 5 or 10 min. A quartz cuvette was used, and the sample analysis volume was fixed as 2.6 mL. In the used cuvette, 0.25 mL of dye (0.32 mM), 0.1 mL of NaBH
4 (0.05 M), water, and AgNP were added according to the values presented in
Table 1.
3. Results
3.1. Synthesis and Characterization of Silver Nanoparticles
AgNPs syntheses and characterizations were performed according to the methodology. For the two different stabilizers used, the data obtained via absorption spectroscopy (
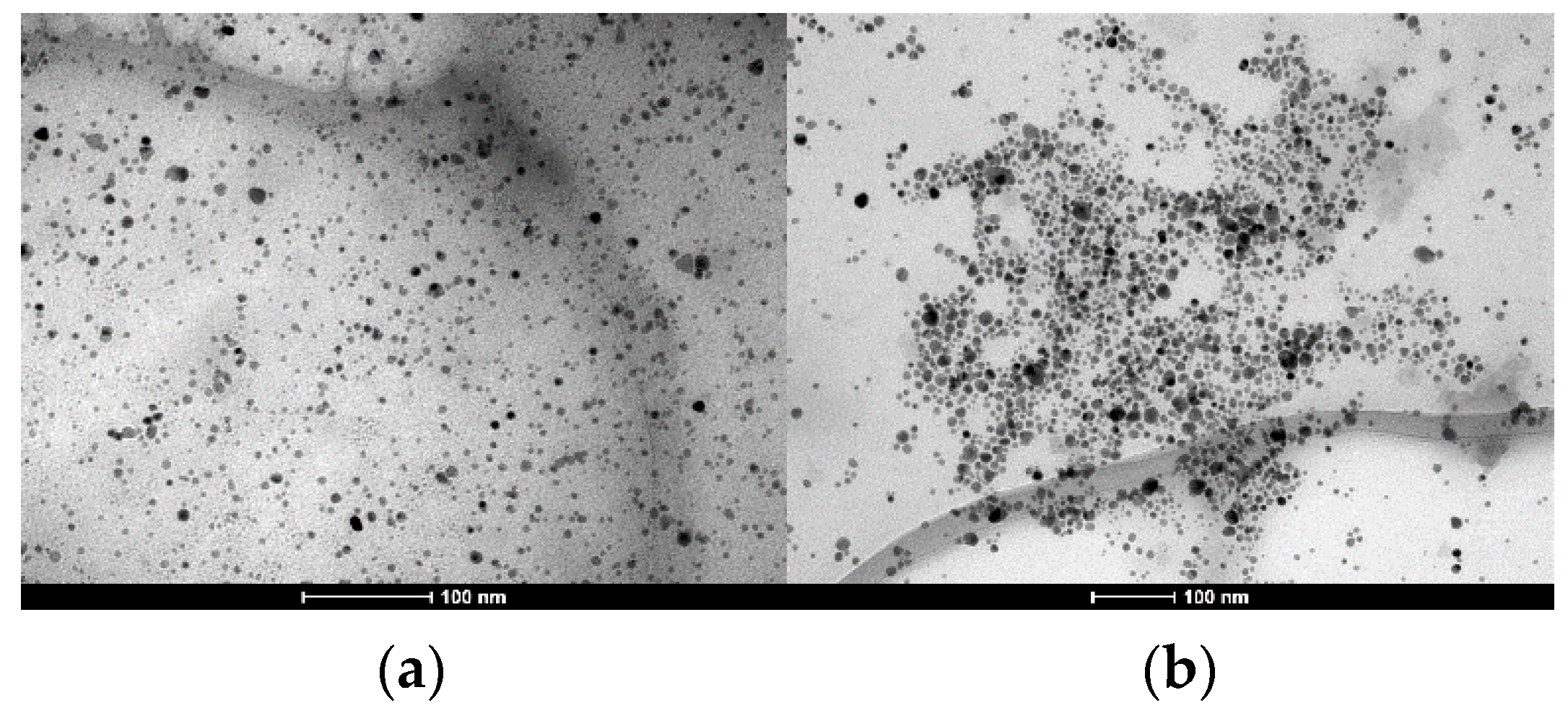
Figure 1) showed a maximum absorption band in 397 nm. From transmission electron microscopy (TEM) (
Figure 1a,b) it was possible to observe the formation of spherical AgNPs with an average diameter of 10.4 ± 4.7 and 28.3 ± 1.1 nm, for AgNPs-PVP and AgNPs-PVA, respectively.
All AgNPs presented Zeta potential values below −30 mV. Using the ICP data, it was possible to calculate the synthesis yield considering the amount of Ag+ added initially. From the values obtained, it appears that the yield of the reaction depends on the stabilizer added. A yield of around 23% and 55% were achieved for AgNPs-PVA and AgNPs-PVP, respectively.
3.2. Catalytic Degradation of Congo Red and Methyl Orange
Before starting the degradation, the absorption spectrum of the dye diluted in water was recorded, serving as a basis for the studies, confirming the value of the absorption bands with maximums at 498 nm and 344 nm for CR, and 464 nm for the MO.
The degradation of CR and MO, using AgNPs-PVA and AgNPs-PVP, was followed by adsorption spectroscopy, and the results are presented in
Figure 2.
4. Discussion
For the two different stabilizers used, the data obtained via absorption spectroscopy and TEM indicate the formation of nanoparticles with spherical shapes. The AgNPs stabilized with PVA presented a higher size than the ones stabilized with PVP. Nevertheless, all the AgNPs were efficient in the degradation of MO and CR in less than 30 min. Regarding the Zeta values, AgNP-PVP presents the most negative value of surface charge, which could not facilitate the electrostatic interaction with the dyes (also anionic). In fact, it did not happen, and this NP proved to be a good catalyst.
With the addition of AgNPs to the dyes, the azo bond (-N=N-) was reduced to an intermediate amine species (-NH-NH-) and, finally, the breaking of these bonds occurs, forming the two degradation products referring to this dye [
3]. AgNPs were successful in the degradation, but the reaction rate was different for the two stabilizers. AgNPs-PVP stands out for performing the “discoloration” faster in the degradation of MO and CR. For the degradation of CR, a much smaller volume of catalyst was used for AgNPs-PVP than for AgNPs-PVA. This catalytic superiority of AgNPs-PVP could be attributed to the higher reaction yield, which implies a greater NPs concentration, and the smaller size, which means a higher reactive surface area.
5. Conclusions
According to the analysis, the nanoparticles showed to be efficient catalysts, obtaining a degradation superior to 80% in 30 min of reaction. AgNP-PVA showed good results in the characterizations and in the degradation process, also being very efficient in the catalytic degradation of the dyes addressed. Thus, it was possible to achieve the main objective of this work and carry out the degradation of MO and CR.
Author Contributions
Conceptualization, N.R.S.M., G.A.L.P. and G.P.; methodology, N.R.S.M., M.T.A.L. and G.P.; validation, N.R.S.M. and G.P.; formal analysis, N.R.S.M.; investigation, N.R.S.M. and M.T.A.L.; writing—original draft preparation, N.R.S.M. and G.P.; writing—review and editing, N.R.S.M., M.T.A.L., G.A.L.P. and G.P.; supervision, G.P.; project administration, G.A.L.P. and G.P.; funding acquisition, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CNPq-Brazil, project ID: 425005/2018-6. N.R.S.M. (IBPG-1107-1.06/22) and M.T.A.L. (IBPG-1071-3.03/22) received a scholarship from FACEPE.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
CNPq: FACEPE, and UFPE.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hanafi, M.F.; Sapawe, N. A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater. Today Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Waghchaurea, R.H.; Vishnu, A.A.; Jagdale, B.S. Photocatalytic degradation of methylene blue, rhodamine B, methyl orange and Eriochrome black T dyes by modified ZnO nanocatalysts: A concise review. Inorg. Chem. Commun. 2022, 143, 109764. [Google Scholar] [CrossRef]
- Cyril, N.; George, J.B.; Joseph, L.; Sylas, V.P. Catalytic degradation of methyl orange and selective sensing of mercury ion in aqueous solutions using green synthesized silver nanoparticles from the seeds of Derris trifoliata. J. Clust. Sci. 2019, 30, 459–468. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).