Polymer/Activated Charcoal-Coated Magnetite for the Adsorptive Removal of Emerging Contaminants: Stepwise Synthesis via Two Sequential Routes †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Nanocomposites via Synthesis Route I
2.3. Preparation of Nanocomposites via Synthesis Route II
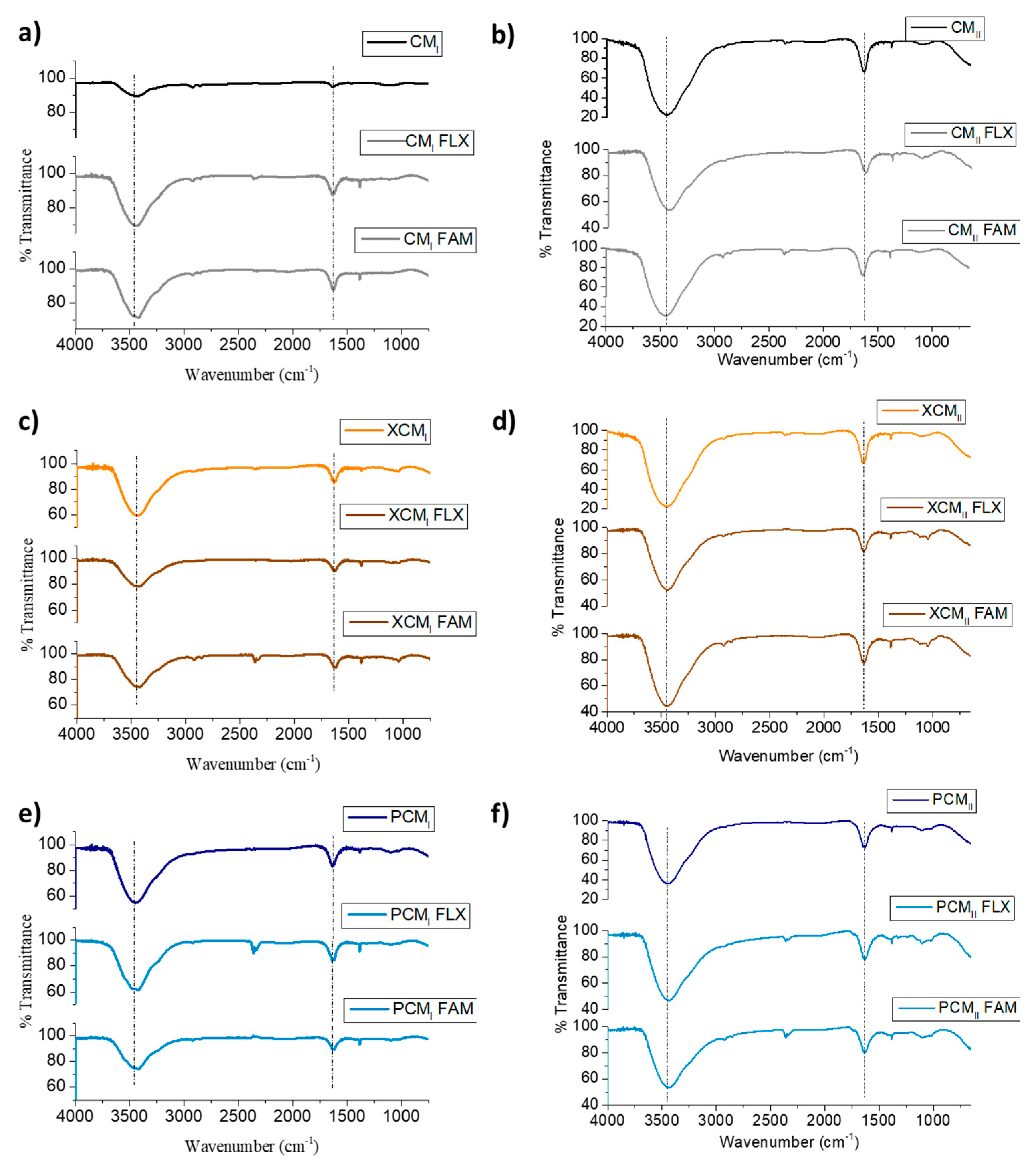
2.4. Characterization of the Prepared Nanocomposites
2.5. Adsorption Experiments
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of the Prepared Nanocomposites
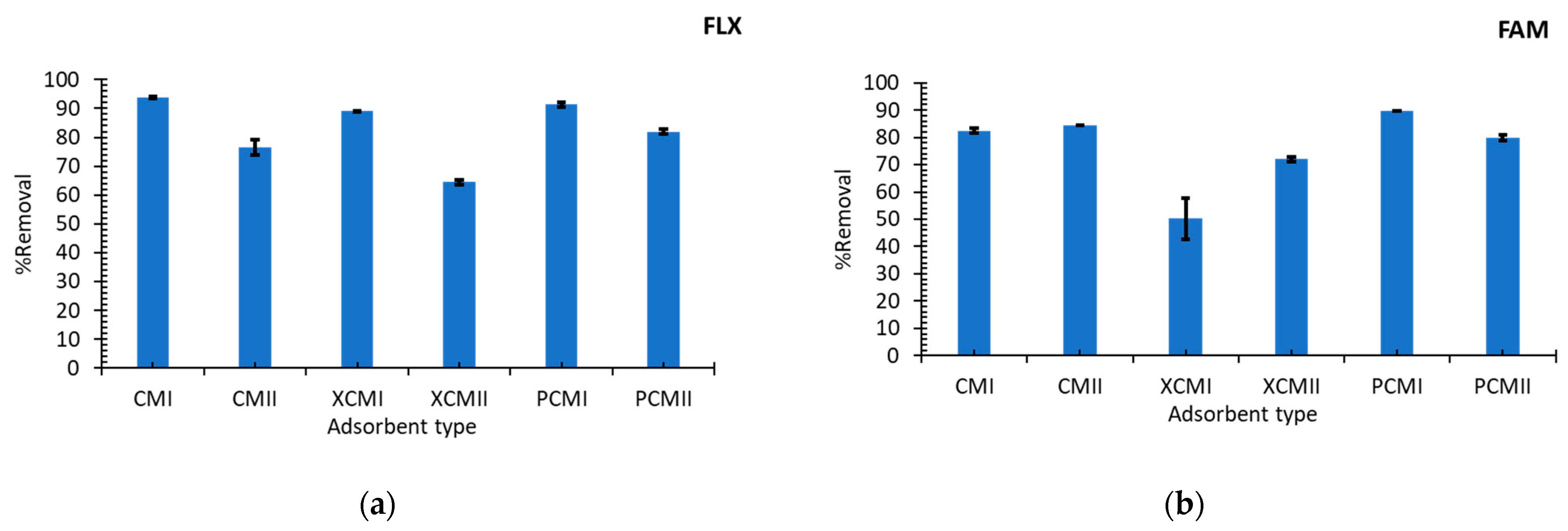
3.2. Adsorption Performance
3.3. Mechanism of Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rathi, B.S.; Kumar, P.S.; Show, P.-L. A review on effective removal of emerging contaminants from aquatic systems: Current trends and scope for further research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.W.; Young, C.J.; Mabury, S.A. Aqueous Photochemical Reaction Kinetics and Transformations of Fluoxetine. Environ. Sci. Technol. 2005, 39, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Sakamoto, H.; Arizono, K.; Shinohara, R. Behavior of Pharmaceuticals in Waste Water Treatment Plant in Japan. Bull. Environ. Contam. Toxicol. 2011, 87, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Foran, C.M.; Richards, S.M.; Weston, J.; Turner, P.K.; Stanley, J.K.; Solomon, K.R.; Slattery, M.; La Point, T.W. Aquatic ecotoxicology of fluoxetine. Toxicol. Lett. 2003, 142, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Afsa, S.; Hamden, K.; Martin, P.A.L.; Ben Mansour, H. Occurrence of 40 pharmaceutically active compounds in hospital and urban wastewaters and their contribution to Mahdia coastal seawater contamination. Environ. Sci. Pollut. Res. 2020, 27, 1941–1955. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Moosavi, S.; Lai, C.W.; Gan, S.; Zamiri, G.; Pivehzhani, O.A.; Johan, M.R. Application of Efficient Magnetic Particles and Activated Carbon for Dye Removal from Wastewater. ACS Omega 2020, 5, 20684–20697. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Martins, M.; Rosca, M.; Rocha, V.; Lago, A.; Neves, I.C.; Tavares, T. Waste-based biosorbents as cost-effective alternatives to commercial adsorbents for the retention of fluoxetine from water. Sep. Purif. Technol. 2020, 235, 116139. [Google Scholar] [CrossRef]
- Rad, T.S.; Khataee, A.; Kayan, B.; Kalderis, D.; Akay, S. Synthesis of pumice-TiO2 nanoflakes for sonocatalytic degradation of famotidine. J. Clean. Prod. 2018, 202, 853–862. [Google Scholar] [CrossRef]
- Omraei, M.; Esfandian, H.; Katal, R.; Ghorbani, M. Study of the removal of Zn(II) from aqueous solution using polypyrrole nanocomposite. Desalination 2011, 271, 248–256. [Google Scholar] [CrossRef]
- Buvaneswari, K.; Singanan, M. Review on scanning electron microscope analysis and adsorption properties of different activated carbon materials. Mater. Today Proc. 2020, in press. [Google Scholar] [CrossRef]
- Sharma, K.; Khaire, K.C.; Thakur, A.; Moholkar, V.S.; Goyal, A. Acacia Xylan as a Substitute for Commercially Available Xylan and Its Application in the Production of Xylooligosaccharides. ACS Omega 2020, 5, 13729–13738. [Google Scholar] [CrossRef] [PubMed]
- Li, F.T.; Yang, H.; Zhao, Y.; Xu, R. Novel modified pectin for heavy metal adsorption. Chin. Chem. Lett. 2007, 18, 325–328. [Google Scholar] [CrossRef]
- Alcaraz, L.; Fernández, A.L.; García-Díaz, I.; López, F.A. Preparation and characterization of activated carbons from winemaking wastes and their adsorption of methylene blue. Adsorpt. Sci. Technol. 2018, 36, 1331–1351. [Google Scholar] [CrossRef]
- Zhang, L.; He, R.; Gu, H.-C. Oleic acid coating on the monodisperse magnetite nanoparticles. Appl. Surf. Sci. 2006, 253, 2611–2617. [Google Scholar] [CrossRef]
- Illés, E.; Tombácz, E. The role of variable surface charge and surface complexation in the adsorption of humic acid on magnetite. Colloids Surf. A Physicochem. Eng. Asp. 2003, 230, 99–109. [Google Scholar] [CrossRef]

| BET Surface Area (m2/g) | BJH Pore Volume (cm3/g) | Pore Size (nm) | Zeta Potential (mV) | |
|---|---|---|---|---|
| CMI | 348.7 | 0.25 | 5.71 | −39.0 |
| CMII | 446.7 | 0.27 | 6.05 | −29.8 |
| XCMI | 227.1 | 0.16 | 6.07 | −30.0 |
| XCMII | 219.8 | 0.13 | 6.33 | −27.8 |
| PCMI | 227.5 | 0.16 | 5.56 | −39.0 |
| PCMII | 266.3 | 0.19 | 7.14 | −37.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farghal, H.H.; Nebsen, M.; El-Sayed, M.M.H. Polymer/Activated Charcoal-Coated Magnetite for the Adsorptive Removal of Emerging Contaminants: Stepwise Synthesis via Two Sequential Routes. Eng. Proc. 2023, 31, 42. https://doi.org/10.3390/ASEC2022-13858
Farghal HH, Nebsen M, El-Sayed MMH. Polymer/Activated Charcoal-Coated Magnetite for the Adsorptive Removal of Emerging Contaminants: Stepwise Synthesis via Two Sequential Routes. Engineering Proceedings. 2023; 31(1):42. https://doi.org/10.3390/ASEC2022-13858
Chicago/Turabian StyleFarghal, Hebatullah H., Marianne Nebsen, and Mayyada M. H. El-Sayed. 2023. "Polymer/Activated Charcoal-Coated Magnetite for the Adsorptive Removal of Emerging Contaminants: Stepwise Synthesis via Two Sequential Routes" Engineering Proceedings 31, no. 1: 42. https://doi.org/10.3390/ASEC2022-13858
APA StyleFarghal, H. H., Nebsen, M., & El-Sayed, M. M. H. (2023). Polymer/Activated Charcoal-Coated Magnetite for the Adsorptive Removal of Emerging Contaminants: Stepwise Synthesis via Two Sequential Routes. Engineering Proceedings, 31(1), 42. https://doi.org/10.3390/ASEC2022-13858






