Effects of Cesium/Formamidinium Co-Addition to Perovskite Solar Cells †
Abstract
1. Introduction
2. Experimental Procedures
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2019, 131, 6050. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–320. [Google Scholar] [CrossRef]
- Oku, T. Crystal structures of perovskite halide compounds used for solar cells. Rev. Adv. Mater. Sci. 2020, 59, 264–305. [Google Scholar] [CrossRef]
- Saliba, M.; Orlandi, S.; Matsui, T.; Aghazada, S.; Cavazzini, M.; Correa-Baena, J.-P.; Gao, P.; Scopelliti, R.; Mosconi, E.; Dahmen, K.H.; et al. A molecularly engineered hole-transporting material for efficient perovskite solar cells. Nat. Energy 2016, 1, 15017. [Google Scholar] [CrossRef]
- Rao, M.K.; Sangeetha, D.N.; Selvakumar, M.; Sudhakar, Y.N.; Mahesha, M.G. Review on persistent challenges of perovskite solar cells’ stability. Sol. Energy 2021, 218, 469–491. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, H.; Yuan, H.; Yang, Z.; Fan, J.Z.; Kim, J.; Voznyy, O.; Gong, X.; Quan, L.N.; Tan, C.S.; et al. Perovskite seeding growth of formamidinium-lead iodide-based perovskites for efficient and stable solar cells. Nat. Commun. 2018, 9, 1607. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Taguchi, M.; Oku, T.; Okita, M.; Minami, S.; Fukunishi, S.; Tachikawa, T. Additive effects of methyl ammonium bromide or formamidinium bromide in methylammonium lead iodide perovskite solar cells using decaphenylcyclopentasilane. J. Mater. Sci. Mater. Electron. 2021, 32, 26449–26464. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef]
- Suzuki, A.; Kato, M.; Ueoka, N.; Oku, T. Additive effect of formamidinium chloride in methylammonium lead halide compound-based perovskite solar cells. J. Electron. Mater. 2019, 48, 3900–3907. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.S.; Wei, S.H.; Berry, J.J.; Zhu, K. Perovskite structures by tuning tolerance factor: Formation of formamidinium and cesium lead iodide solid-state alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, C.; Grice, C.R.; Shrestha, N.; Chen, J.; Zhao, D.; Liao, W.; Cimaroli, A.J.; Roland, P.J.; Ellingson, R.J.; et al. Improving the performance of formamidinium and cesium lead triiodide perovskite solar cells using lead thiocyanate additives. ChemSusChem 2016, 9, 3288–3297. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T.; Suzuki, A.; Sakamoto, H.; Yamada, M.; Minami, S.; Miyauchi, S. Fabrication and characterization of CH3NH3(Cs)Pb(Sn)I3(Cl) perovskite solar cells with TiO2 nanoparticle layers. Jpn. J. Appl. Phys. 2018, 57, 02CE03. [Google Scholar] [CrossRef]
- Zhang, M.; Yun, J.S.; Ma, Q.; Zheng, J.; Lau, C.F.J.; Deng, X.; Kim, J.; Kim, D.; Seidel, J.; Green, M.A.; et al. High-efficiency rubidium-incorporated perovskite solar cells by gas quenching. ACS Energy Lett. 2017, 2, 438–444. [Google Scholar] [CrossRef]
- Hu, Y.; Hutter, E.M.; Rieder, P.; Grill, I.; Hanisch, J.; Aygüler, M.F.; Hufnagel, A.G.; Handloser, M.; Bein, T.; Hartschuh, A.; et al. Understanding the role of cesium and rubidium additives in perovskite solar cells: Trap states, charge transport, and recombination. Adv. Energy Mater. 2018, 8, 1703057. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T.; Suzuki, A. Effects of doping with Na, K, Rb, and formamidinium cations on (CH3NH3)0.99Rb0.01Pb0.99Cu0.01I3−x(Cl, Br)x perovskite photovoltaic cells. AIP Adv. 2020, 10, 125023. [Google Scholar] [CrossRef]
- Takada, K.; Oku, T.; Suzuki, A.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Fabrication and characterization of ethylammonium- and rubidium-added perovskite solar cells. Chem. Proc. 2022, 9, 14. [Google Scholar] [CrossRef]
- Zheng, F.; Chen, W.; Bu, T.; Ghiggino, K.P.; Huang, F.; Cheng, Y.; Tapping, P.; Kee, T.W.; Jia, B.; Wen, X. Triggering the passivation effect of potassium doping in mixed-cation mixed-halide perovskite by light illumination. Adv. Energy Mater. 2019, 9, 1901016. [Google Scholar] [CrossRef]
- Kandori, S.; Oku, T.; Nishi, K.; Kishimoto, T.; Ueoka, N.; Suzuki, A. Fabrication and characterization of potassium- and formamidinium-added perovskite solar cells. J. Ceram. Soc. Jpn. 2020, 128, 805. [Google Scholar] [CrossRef]
- Machiba, H.; Oku, T.; Kishimoto, T.; Ueoka, N.; Suzuki, A. Fabrication and evaluation of K-doped MA0.8FA0.1K0.1PbI3(Cl) perovskite solar cells. Chem. Phys. Lett. 2019, 730, 117–123. [Google Scholar] [CrossRef]
- Oku, T.; Kandori, S.; Taguchi, M.; Suzuki, A.; Okita, M.; Minami, S.; Fukunishi, S.; Tachikawa, T. Polysilane-inserted methylammonium lead iodide perovskite solar cells doped with formamidinium and potassium. Energies 2020, 13, 4776. [Google Scholar] [CrossRef]
- Enomoto, A.; Suzuki, A.; Oku, T.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Effects of Cu, K and guanidinium addition to CH3NH3PbI3 perovskite solar cells. J. Electron. Mater. 2022, 51, 4317–4328. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T. Effects of co-addition of sodium chloride and copper(ii) bromide to mixed-cation mixed-halide perovskite photovoltaic devices. ACS Appl. Energy Mater. 2020, 9, 24231. [Google Scholar] [CrossRef]
- Okumura, R.; Oku, T.; Suzuki, A.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Effects of adding alkali metals and organic cations to Cu-based perovskite solar cells. Appl. Sci. 2022, 12, 1710. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T.; Suzuki, A. Additive effects of alkali metals on Cu-modified CH3NH3PbI3−δClδ photovoltaic devices. RSC Adv. 2019, 9, 24231. [Google Scholar] [CrossRef]
- Suzuki, A.; Kitagawa, K.; Oku, T.; Okita, M.; Fukunishi, S.; Tachikawa, T. Additive effects of copper and alkali metal halides into methylammonium lead iodide perovskite solar cells. Electron. Mater. Lett. 2022, 18, 176–186. [Google Scholar] [CrossRef]
- Tosado, G.A.; Zheng, E.; Yu, Q. Tuning cesium–guanidinium in formamidinium tin triiodide perovskites with an ethylenediammonium additive for efficient and stable lead-free perovskite solar cells. Mater. Adv. 2020, 1, 3507–3517. [Google Scholar] [CrossRef]
- Kishimoto, T.; Suzuki, A.; Ueoka, N.; Oku, T. Effects of guanidinium addition to CH3NH3PbI3-xClx perovskite photovoltaic devices. J. Ceram. Soc. Jpn. 2019, 127, 491–497. [Google Scholar] [CrossRef]
- Kishimoto, T.; Oku, T.; Suzuki, A.; Ueoka, N. Additive effects of guanidinium iodide on CH3NH3PbI3 perovskite solar cells. Phys. Status Solidi A 2021, 218, 2100396. [Google Scholar] [CrossRef]
- Ono, I.; Oku, T.; Suzuki, A.; Asakawa, Y.; Terada, S.; Okita, M.; Fukunishi, S.; Tachikawa, T. Fabrication and characterization of CH3NH3PbI3 solar cells with added guanidinium and inserted with decaphenylpentasilane. Jpn. J. Appl. Phys. 2022, 61, SB1024. [Google Scholar] [CrossRef]
- Nishi, K.; Oku, T.; Kishimoto, T.; Ueoka, N.; Suzuki, A. Photovoltaic characteristics of CH3NH3PbI3 perovskite solar cells added with ethylammonium bromide and formamidinium iodide. Coatings 2020, 10, 410. [Google Scholar] [CrossRef]
- Terada, S.; Oku, T.; Suzuki, A.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Ethylammonium bromide- and potassium-added CH3NH3PbI3 perovskite solar cells. Photonics 2022, 9, 791. [Google Scholar] [CrossRef]
- Taguchi, M.; Suzuki, A.; Oku, T.; Fukunishi, S.; Minami, S.; Okita, M. Effects of decaphenylcyclopentasilane addition on photovoltaic properties of perovskite solar cells. Coatings 2018, 8, 461. [Google Scholar] [CrossRef]
- Oku, T.; Nomura, J.; Suzuki, A.; Tanaka, H.; Fukunishi, S.; Minami, S.; Tsukada, S. Fabrication and characterization of CH3NH3PbI3 perovskite solar cells added with polysilanes. Int. J. Photoenergy 2018, 2018, 8654963. [Google Scholar] [CrossRef]
- Taguchi, M.; Suzuki, A.; Oku, T.; Ueoka, N.; Minami, S.; Okita, M. Effects of annealing temperature on decaphenylcyclopentasilane-inserted CH3NH3PbI3 perovskite solar cells. Chem. Phys. Lett. 2019, 737, 136822. [Google Scholar] [CrossRef]
- Oku, T.; Nakagawa, J.; Iwase, M.; Kawashima, A.; Yoshida, K.; Suzuki, A.; Akiyama, T.; Tokumitsu, K.; Yamada, M.; Nakamura, M. Microstructures and photovoltaic properties of polysilane-based solar cells. Jpn. J. Appl. Phys. 2013, 52, 04CR07. [Google Scholar] [CrossRef]
- Oku, T.; Taguchi, M.; Suzuki, A.; Kitagawa, K.; Asakawa, Y.; Yoshida, S.; Okita, M.; Minami, S.; Fukunishi, S.; Tachikawa, T. Effects of polysilane addition to chlorobenzene and high temperature annealing on CH3NH3PbI3 perovskite photovoltaic devices. Coatings 2021, 11, 665. [Google Scholar] [CrossRef]
- Mizuno, S.; Oku, T.; Suzuki, A.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Photovoltaic properties and microstructures of polysilane-added perovskite solar cells. Chem. Proc. 2022, 9, 20. [Google Scholar] [CrossRef]
- Oku, T.; Zushi, M.; Imanishi, Y.; Suzuki, A.; Suzuki, K. Microstructures and photovoltaic properties of perovskite-type CH3NH3PbI3 compounds. Appl. Phys. Express 2014, 7, 121601. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T. Stability Characterization of PbI2-added CH3NH3PbI3−xClx photovoltaic devices. ACS Appl. Mater. Interfaces 2018, 10, 44443–44451. [Google Scholar] [CrossRef] [PubMed]
- Oku, T.; Ohishi, Y.; Ueoka, N. Highly (100)-oriented CH3NH3PbI3(Cl) perovskite solar cells prepared with NH4Cl using an air blow method. RSC Adv. 2018, 8, 10389–10395. [Google Scholar] [CrossRef] [PubMed]
- Oku, T.; Ohishi, Y. Effects of annealing on CH3NH3PbI3 (Cl) perovskite photovoltaic devices. J. Ceram. Soc. Jpn. 2018, 126, 56–60. [Google Scholar] [CrossRef]
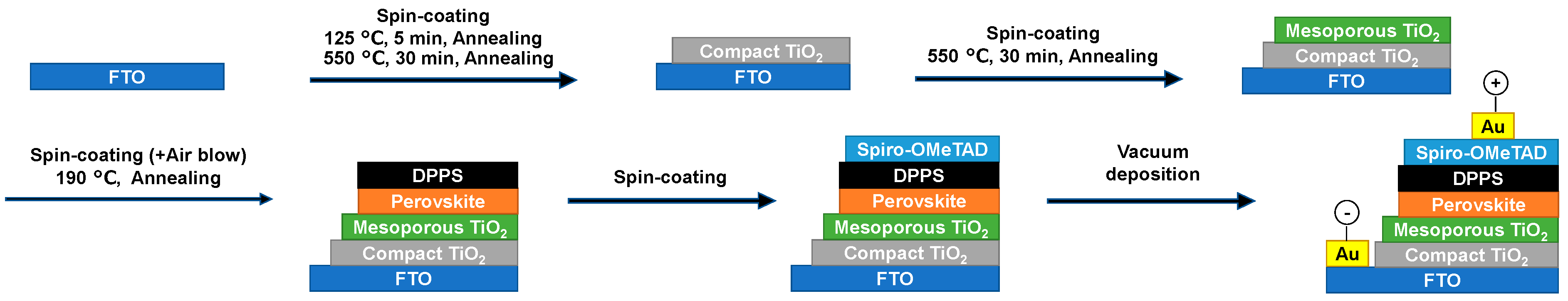
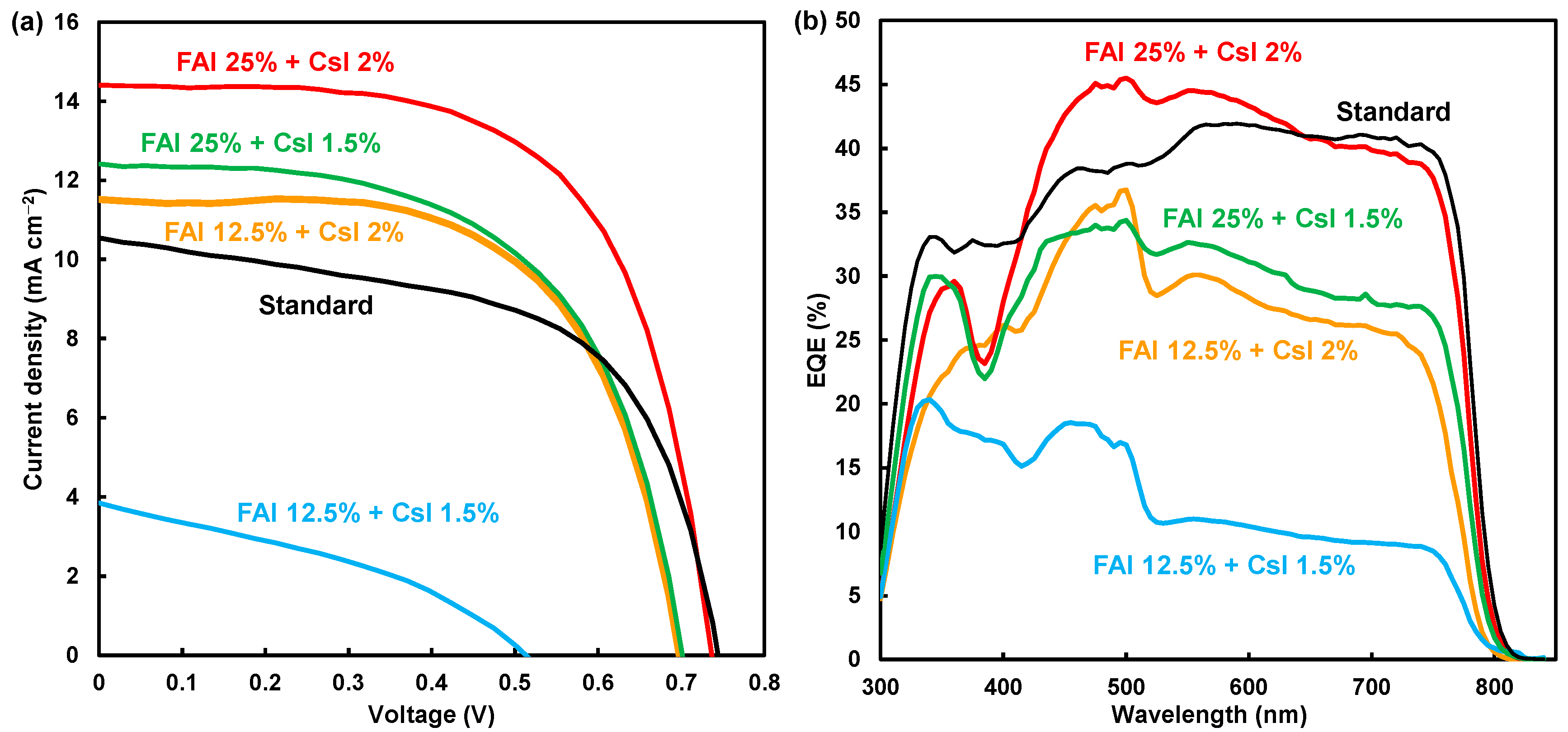
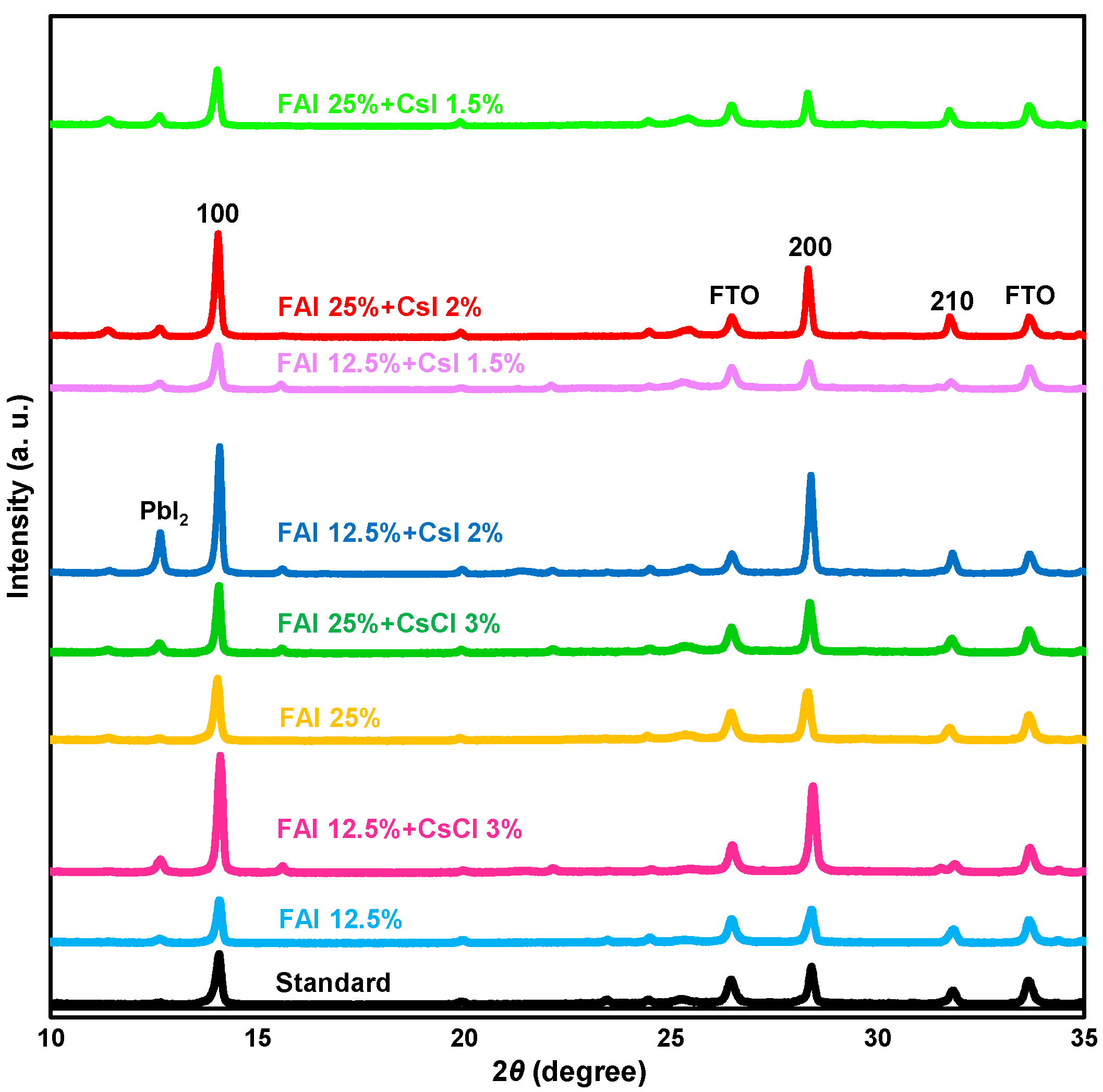
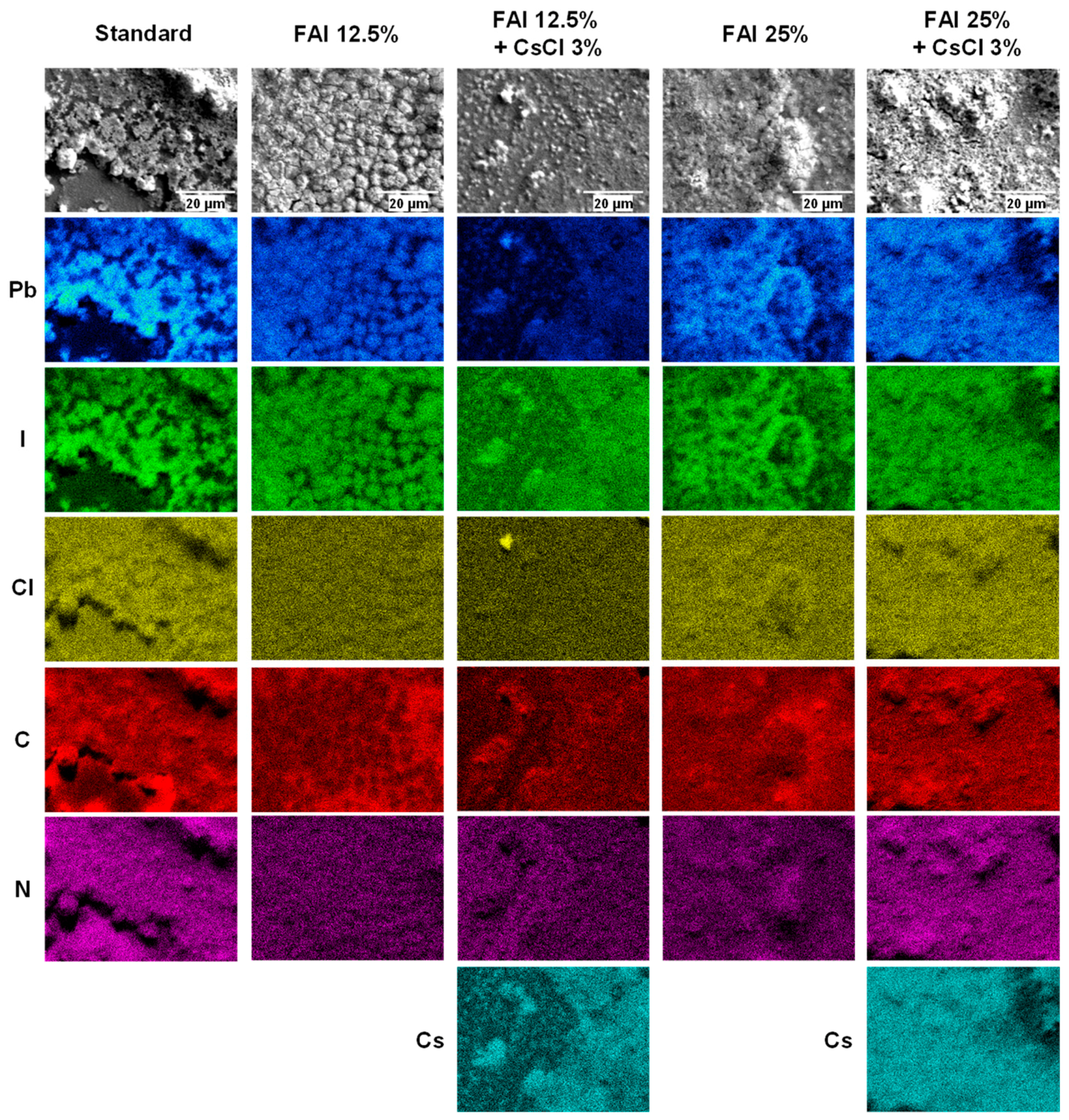
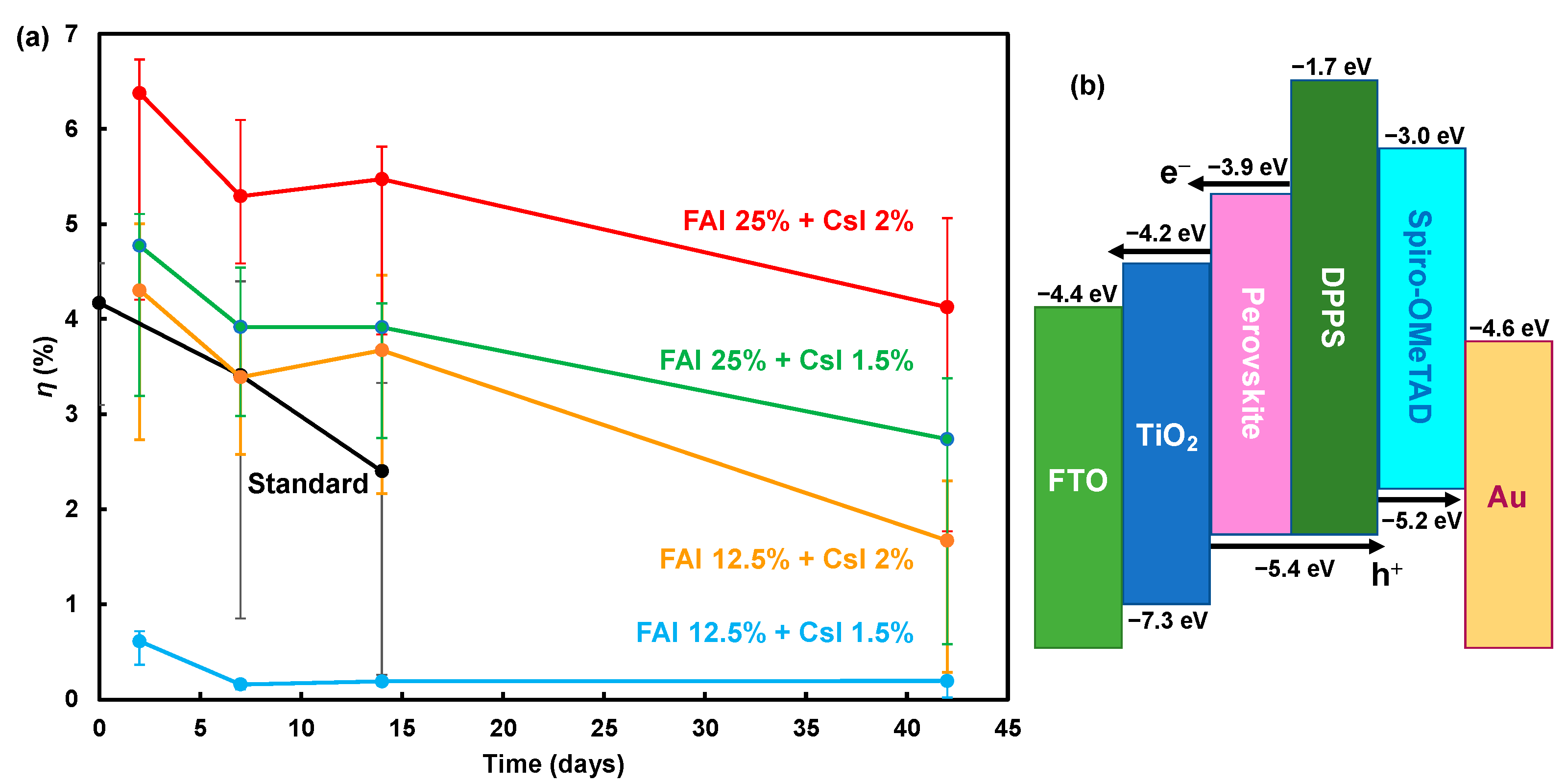
| Device | JSC (mA cm−2) | VOC (V) | FF | RS (Ω cm2) | RSh (Ω cm2) | η (%) | ηave (%) | Eg (eV) | |
|---|---|---|---|---|---|---|---|---|---|
| FAI (%) | CsI (%) | ||||||||
| 0 | 0 | 10.55 | 0.744 | 0.585 | 1.49 | 325 | 4.59 | 4.17 | 1.544 |
| 12.5 | 1.5 | 3.84 | 0.513 | 0.364 | 2.26 | 224 | 0.72 | 0.61 | 1.554 |
| 12.5 | 2 | 11.52 | 0.698 | 0.623 | 1.90 | 8200 | 5.00 | 4.30 | 1.563 |
| 25 | 1.5 | 12.41 | 0.701 | 0.587 | 1.68 | 2654 | 5.11 | 4.77 | 1.549 |
| 25 | 2 | 14.41 | 0.737 | 0.634 | 1.71 | 10,338 | 6.73 | 6.38 | 1.548 |
| Device | Lattice Constant (Å) | Crystallite Size (Å) | Orientation I100/I210 | ||
|---|---|---|---|---|---|
| FAI (%) | CsCI (%) | CsI (%) | |||
| 0 | 0 | 0 | 6.279(2) | 590 | 2.99 |
| 12.5 | 0 | 0 | 6.278(2) | 671 | 2.62 |
| 12.5 | 3 | 0 | 6.272(2) | 631 | 9.67 |
| 25 | 0 | 0 | 6.299(1) | 579 | 3.84 |
| 25 | 3 | 0 | 6.286(1) | 876 | 3.81 |
| 12.5 | 0 | 1.5 | 6.290(3) | 545 | 4.46 |
| 12.5 | 0 | 2 | 6.282(0) | 762 | 5.16 |
| 25 | 0 | 1.5 | 6.299(2) | 592 | 3.05 |
| 25 | 0 | 2 | 6.297(1) | 657 | 4.46 |
| Device | Pb (at%) | I (at%) | Cl (at%) | C:N (at%) | |
|---|---|---|---|---|---|
| FAI (%) | CsCl (%) | ||||
| 0 | 0 | 30.3 | 57.0 | 12.7 | 38.6:61.4 |
| 12.5 | 0 | 29.7 | 57.7 | 12.7 | 48.3:51.7 |
| 12.5 | 3 | 39.4 | 32.1 | 28.4 | 33.7:66.3 |
| 25 | 0 | 30.0 | 59.1 | 10.9 | 44.8:55.2 |
| 25 | 3 | 27.4 | 63.0 | 9.57 | 36.6:63.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nonomura, R.; Oku, T.; Ono, I.; Suzuki, A.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Effects of Cesium/Formamidinium Co-Addition to Perovskite Solar Cells. Eng. Proc. 2023, 31, 32. https://doi.org/10.3390/ASEC2022-13789
Nonomura R, Oku T, Ono I, Suzuki A, Okita M, Fukunishi S, Tachikawa T, Hasegawa T. Effects of Cesium/Formamidinium Co-Addition to Perovskite Solar Cells. Engineering Proceedings. 2023; 31(1):32. https://doi.org/10.3390/ASEC2022-13789
Chicago/Turabian StyleNonomura, Ren, Takeo Oku, Iori Ono, Atsushi Suzuki, Masanobu Okita, Sakiko Fukunishi, Tomoharu Tachikawa, and Tomoya Hasegawa. 2023. "Effects of Cesium/Formamidinium Co-Addition to Perovskite Solar Cells" Engineering Proceedings 31, no. 1: 32. https://doi.org/10.3390/ASEC2022-13789
APA StyleNonomura, R., Oku, T., Ono, I., Suzuki, A., Okita, M., Fukunishi, S., Tachikawa, T., & Hasegawa, T. (2023). Effects of Cesium/Formamidinium Co-Addition to Perovskite Solar Cells. Engineering Proceedings, 31(1), 32. https://doi.org/10.3390/ASEC2022-13789






