Abstract
With hydrogen as one of the energetic vectors craved for use in the future, the successful de-carbonization of the energy sector will require an increase in hydrogen production from renewable resources. Materials that are able to catalyze the water-splitting reaction through sunlight absorption have been widely studied as an adequate solution for green hydrogen generation. Among the proposed tantalum-based oxide materials, Sr2Ta2O7 displays moderate photocatalytic activity. Aiming to improve the photocatalytic properties by means of compositional modifications, this work presents a DFT study of the Sr substitution with Ca. The structural, energetic, and electronic features of the phases of CaxSr2−xTa2O7 (0 < x < 1) have been examined. The computational results utilizing the SCAN functional show that there is a slight decrement in the band gap value (from 3.65 eV for x = 0 to 3.50 eV for x = 1) concomitant to a minor distortion of the crystal structure.
1. Introduction
Climate change might be one of the biggest challenges that we face nowadays as a society. Currently, most of the produced energy comes from fossil fuels, which contributes to greenhouse gas emissions. Energy production through renewable sources is being explored as a solution to this major issue. In that respect, hydrogen (H2) seems to be the energetic vector of the future [1,2]; it presents a high combustion efficiency and the highest energy content per unit mass of all fossil fuels normally used [3]. However, hydrogen production rarely comes from zero-emission sources. In this sense, hydrogen production through water splitting using sunlight (i.e., photocatalysis) presents a key to green hydrogen generation [4] (p. 3).
The photocatalytic dissociation of water can be achieved using semiconductor materials that are able to absorb sunlight, leading to the promotion of electrons from the valence band (VB) to the conduction band (CB). There are, however, some vital concerns that must be considered: the VB maximum should be below the O2/H2O oxidation potential (1.23 eV) and the CB minimum should be above the H2O/H2 reduction potential (0.0 eV). Likewise, the band gap should be smaller than 3.0 eV to use visible light and larger than 1.23 eV to fulfill band position requirements [5]. Several compounds have been studied for this purpose, for example, TiO2, WO3, CdSe, CdS, and Ta2O5 [6]. Among the investigated d0-TM oxides, Sr2Ta2O7 [7,8] shows some exciting characteristics, particularly in its CB position relative to H2O/H2 reduction potential. However, its large band gap (4.6 eV) limits the light absorption to the UV region of sunlight.
The Sr2Ta2O7 compound presents a perovskite-derived structure [9], where four perovskite layers form 2D nets perpendicular to the c axis, as shown in Figure 1. In the ABO3 perovskite blocks, Ta occupies the B position, being coordinated to six O atoms. Sr occupies two different positions, Sr(1) and Sr(2), that are coordinated to 8 and 12 O atoms, respectively. Sr(1) can be described as an atom between perovskite block layers, while Sr(2) can be seen as an atom inside those blocks.
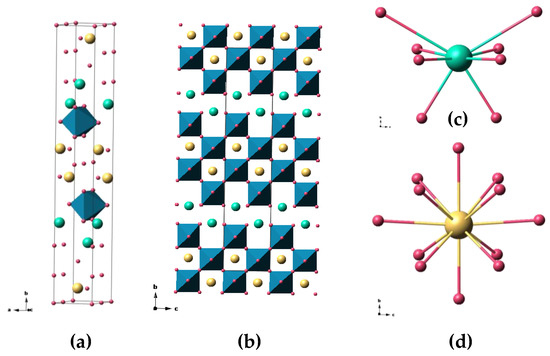
Figure 1.
(a) Crystallographic unit cell of Sr2Ta2O7 [S.G. Cmcm (No.63)]; (b) representation of the layered structure showing the perovskite blocks; (c) detail of the Sr(1) coordination environment; and (d) detail of Sr(2) coordination environment. Color code: Ta atoms are shown in blue, O in red, Sr(1) in green, and Sr(2) in yellow.
Previous computational studies [10,11] suggest that anion modifications along with cation doping in the Ta site tune the band gap value and the relative positions of the VB/CB, thereby influencing the photocatalytic activity As for cation doping in the Sr site, some authors [12] have experimentally demonstrated that the partial substitution of Sr for Ba improves the photocatalytic activity. Specifically, the solid solution Sr2−xBaxTa2O7 was successfully synthesized in the compositional range 0 < x < 0.4, with the term x = 0.4 displaying the best catalytic performance. This makes the investigation of the substitution of Sr with other cations such as Ca, appealing in that it could potentially enhance the photocatalytic activity of Sr2Ta2O7. For this purpose, this work focuses on the computational study through DFT calculations of the structural and electronic changes that the substitution of Sr with Ca can provoke in the CaxSr2−xTa2O7 family. In particular, the x = 0 and 1 phases are studied.
2. Materials and Methods
2.1. Crystallographic Models
Crystallographic models were built from the Sr2Ta2O7 structure (ICSD file 601) [9]. The structure was used to model the hypothetical Sr2−xCaxTa2O7 compound with, x = 1. However, since there are two possible crystallographic sites for the Sr substitution (Figure 1), two models of CaSrTa2O7 were constructed. The substitution of Sr(1) for Ca led to the model named CaSrTa2O7(1), and the substitution of Sr(2) for Ca generated the model named CaSrTa2O7(2).
2.2. Computational Methods
DFT calculations were performed using the VASP package (Vienna ab-initio simulation package) developed at the Universität Wien [13,14]. The Projector Augmented Wave (PAW) [15] method was used to describe the interaction of core electrons with nuclei, specifically 4s24p65s2 for Sr, 5p66s25d3 for Ta, 2s22p4 for O, and 3s23p64s2 for Ca were treated as valence electrons. A meta-GGA exchange-correlation functional was utilized; in particular, the recently developed strongly constrained and appropriately normed (SCAN) functional [16]. The energy cutoff was set at 600 eV throughout all calculations. Integration of the first Brillouin zone was carried out under the determination of k-points by the Monkhorts–Pack scheme. The k-point meshes were set at 6 × 2 × 6 in all cases, along with a Gaussian smearing parameter of 0.05 eV. For the density of states (DOS) calculations, the tetrahedron method with Bloch corrections [17] was employed. The tolerance threshold in total energy to achieve self-consistency was set at 1 × 10−4 eV. Structural relaxation was performed over atoms’ position, as well as cell shape and volume. No symmetry constraints were imposed during a relaxation for the Ca models calculations.
3. Results and Discussion
3.1. Crystal Structure
The structural optimization results for Sr2Ta2O7 within the SCAN functional are shown in Table 1. The lattice parameters obtained are in good agreement with experimental measurements [9], with errors below 0.5%. Likewise, distances results are, in general, in line with experimental values, showing deviations below 4.5%. Thus, the SCAN functional correctly reproduces the crystal structure of Sr2Ta2O7.

Table 1.
Lattice parameters and atomic distances in the Sr2Ta2O7 (S.G. Cmcm) structure and CaSrTa2O7 models (S.G. Cmcm).
Regarding the relative stability of the two crystallographic models proposed for CaSrTa2O7, DFT calculations show that the CaSrTa2O7(1) model has lower energy than the CaSrTa2O7(2) model (energy difference of 332 meV). The relative stability can be rationalized considering the ionic radii of Ca2+ (r = 1.12 Å for C.N. = 8 and 1.34 Å for C.N. = 12 [18]) and Sr2+ (r = 1.26 Å for C.N. = 8 and 1.44 Å for C.N. = 12 [18]). This calculated energy indicates that a more stable structure is formed if the larger Sr2+ occupies the position inside perovskite blocks (C.N. = 12), which is to say, the smaller Ca2+ cation will tend to occupy the site between perovskite layers position (C.N. = 8).
Lattice parameters and atomic distances for the most stable CaSrTa2O7 model are listed in Table 1. As expected, the introduction of the smaller Ca2+ ion produces a contraction of the unit cell and a reduction in all lattice parameters. Overall, the introduction of Ca ions preserves the initial crystal structure of Sr2Ta2O7.
3.2. Electronic Structure
Figure 2 and Figure 3 show the calculated density of states and band structure of the Sr2Ta2O7 and DOS of the two CaSrTa2O7 models, respectively.
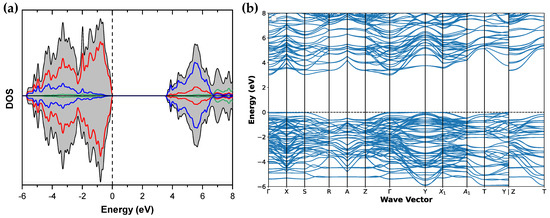
Figure 2.
(a) Calculated density of states of Sr2Ta2O7. (b) Calculated band structure of Sr2Ta2O7. The Fermi level is set at the zero of energy. Color code: total black, O red, Sr green, and Ta blue.
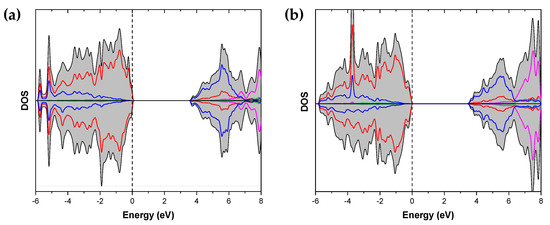
Figure 3.
Calculated density of states of (a) CaSrTa2O7(1) and (b) CaSrTa2O7(2). The Fermi level is set at the zero of energy. Color code: total black, O red, Sr green, Ca pink, Ta blue.
The DOS for Sr2Ta2O7 is represented in Figure 2a. The Fermi level is set at the 0 of energy. The VB is mainly formed by the 2p states of O while CB is composed of the 5d Ta empty states (in blue). Yet, a good hybridization, signature of Ta-O covalence, exists between O and Ta states in both bands. Sr barely contributes to forming the valence and conduction bands. As shown in Figure 2a,b, band gap of 3.65 eV is obtained using the SCAN functional. This result deviates from the experimental one (4.6 eV), given that band gap underestimation is a known failure of DFT methods [19].
Despite the limitations of DFT to reproduce accurate band-gap values, the trends in band-gaps due to chemical composition modifications are fully reliable [11].
Figure 3 shows the calculated DOS for the two models used to simulate the CaSrTa2O7 compounds. CaSrTa2O7(1) has a band gap of 3.55 eV while the CaSrTa2O7(2) band gap is 3.50 eV. Therefore, calculations suggest that in the Sr2−xCaxTa2O7 family, the trend is to reduce the band gap as x increases. Importantly, band gap variations are of only about 0.15 eV when going from x = 0 to x = 1. It is well documented that in d0 transition metals oxides crystallizing in the ABO3 perovskite structure, the A substitution with other ions can provoke a distortion in the crystal structure, which could ultimately affect the electronic structure producing significant band-gap values modifications [20]. Since, in the present case, the partial substitution of Sr with Ca causes minor crystal structure modifications, the variations in the electronic structure are subtle. Additionally, valence and conduction bands in CaSrTa2O7 models have essentially the same features as in the Sr2Ta2O7 phase.
Considering the exposed results, the substitution of Sr with Ca in Sr2Ta2O7 only leads to a slight reduction in the band gap value that is independent of the Ca position. This seems insufficient to cause an effective change in the photocatalytic activity of the material. Taking into account the experimentally observed improvement of photocatalytic activity when substituting Sr with Ba in some Sr2−xBaxTa2O7 phases [12], more work is needed to assess the impact of the Sr substitution in Sr2Ca2O7 with Ca/Ba and other cations in the electronic structure. It should be highlighted that other critical features could account for an improved catalytic activity in Sr2−xBaxTa2O7, such as the decrement of the electron-hole recombination.
4. Conclusions
Computational investigations at the level of Density Functional Theory (DFT) permit the prediction of some basic and critical features in view of the photocatalytic activity of materials. In this work, DFT methods are used to predict the crystal and electronic structures of the potential photocatalyst Sr2−xCaxTa2O7. We found that the x = 1 phases preserve the perovskite-related structure of the parent Sr2Ta2O7. The calculated DOS shows a band narrowing of 0.10–0.15 eV because of the Sr substitution with Ca. With band gaps of ~4.5 eV, sunlight absorption by the SrCaTa2O7 photocatalyst is limited to the UV region. Work is in progress to investigate other substitutions that may enhance the photocatalytic activity of Sr2Ta2O7.
Author Contributions
M.G.-T.: methodology, formal analysis, investigation, data curation, writing-original draft preparation, visualization. K.B.: conceptualization, validation, resources. M.E.A.-d.D.: conceptualization, supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
Universidad Complutense de Madrid (FEI-EU-22-01-4129585) and Comunidad de Madrid (PEJ-2020-AI/IND-18065 under the program Ayudas para la contratación de Ayudantes de Investigación y Técnicos de Laboratorio).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the plots within this work and other findings of this study are available from the corresponding authors upon reasonable request.
Acknowledgments
Authors thank I. Collado and R. Carrasco for their contributions to the experimental data analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrog. Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrog. Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Hydrogen Production. Compr. Energy Syst. 2018, 3–5, 1–40. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, F.E. Inorganic materials as catalysts for photochemical splitting of water. Chem. Mater. 2008, 20, 35–54. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. New tantalate photocatalysts for water decomposition into H2 and O2. Chem. Phys. Lett. 1998, 295, 487–492. [Google Scholar] [CrossRef]
- Kudo, A.; Kato, H.; Nakagawa, S. Water Splitting into H2 and O2 on New Sr2M2O7 (M = Nb and Ta) Photocatalysts with Layered Perovskite Structures: Factors Affecting the Photocatalytic Activity. J. Phys. Chem. B 2000, 104, 571–575. [Google Scholar] [CrossRef]
- Ishizawa, N.M.F.; Kawamura, T.; Kimura, M. Compounds with Perovskite-Type Slabs. II. The Crystal Structure of Sr2Ta207. Acta Crystallogr. Sect. B 1976, B32, 2564–2566. [Google Scholar] [CrossRef]
- Liu, P.; Nisar, J.; Ahuja, R.; Pathak, B. Layered perovskite Sr2Ta2O7 for visible light photocatalysis: A first principles study. J. Phys. Chem. C 2013, 117, 5043–5050. [Google Scholar] [CrossRef]
- Peng, Y.; Ma, Z.; Hu, J.; Wu, K. A first-principles study of anionic (S) and cationic (V/Nb) doped Sr2Ta2O7 for visible light photocatalysis. RSC Adv. 2017, 7, 40922–40928. [Google Scholar] [CrossRef]
- Kim, K.Y.; Eun, T.H.; Lee, S.S.; Chon, U. Photocatalytic Activities and Structural Changes of Barium-Doped Strontium Tantalate. Resour. Process. 2009, 56, 138–144. [Google Scholar] [CrossRef]
- Furthmüller, G.K.J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Bloch, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ruzsinszky, A.; Perdew, J.P. Strongly Constrained and Appropriately Normed Semilocal Density Functional. Phys. Rev. Lett. 2015, 115, 036402. [Google Scholar] [CrossRef] [PubMed]
- Blochl, P.E.; Jepsen, O.; Andersen, O.K. Improved Tetrahedron Method for Brillouin-Zone integrations. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Crystallogr. A 1976, 32, 751. [Google Scholar] [CrossRef]
- Borlido, P.; Schmidt, J.; Huran, A.W.; Tran, F.; Marques, M.A.L.; Botti, S. Exchange-correlation functionals for band gaps of solids: Benchmark, reparametrization and machine learning. NPJ Comput. Mater. 2020, 6, 96. [Google Scholar] [CrossRef]
- Eng, H.W.; Barnes, P.W.; Auer, B.M.; Woodward, P.M. Investigations of the electronic structure of d0 transition metal oxides belonging to the perovskite family. J. Solid State Chem. 2003, 175, 94–109. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).