Abstract
Electrospun polymer-based nanofibers are of increasing interest in contemporary applied biomedicine. The challenge regarding modern surgery and tissue engineering is to discover a variety of manufactured scaffolds with improved properties that can replace and regenerate damaged skin and organs. The unique properties of polymer nanofibers, such as submicron and nanoscale diameters, large surface area, flexibility, etc., make them attractive objects for a wide range of applications. In this study, a combination of chitosan as a natural polymer and poly(lactic) acid as a synthetic polymer is studied with the aim of improving and accelerating the healing of skin wounds. Chitosan (Chi) is one of the most promising polymers for scaffold design, due to its high biodegradability, non-toxic, and antibacterial properties. On the other hand, poly(lactic) acid (PLA) possesses enhanced electrospinability potential and desirable mechanical strength. Therefore, the combination of Chi and PLA enhances the mutually superior properties of both. After optimizing the process parameters, imaging, and determining the diameter of the nanofibers, the scaffold potential for wound healing was investigated by in vitro scratch test on a healthy fibroblast cell line. The study concludes that ultrafine Chi:PLA nanofiber scaffolds have significant potential to regenerate and restore damaged tissue under in vitro conditions.
1. Introduction
Relatively rapid wound healing is a time factor that significantly contributes to the improvement of modern humanity’s quality of life. A shorter recovery period from injuries contributes to shorter hospital stays, reduced needs for hospital resources, fewer infections, and faster returns of patients to regular work and other life activities. Scientific breakthroughs in regenerative medicine are therefore critical to the effective and high-quality functioning of modern society.
Regenerative medicine is increasingly focusing on techniques for creating nanofibers that mimic the natural structures of tissues and organs. In this regard, electrospinning is one of the most widely used techniques. This cutting-edge bioengineering technique generates nanofibers from synthetic or natural polymers. The resulting polymers form matrices (scaffolds) with optimal properties for imitating natural tissues and are used for tissue regeneration and/or organ replacement.
The polymer materials are commonly used to create multifunctional matrices for wound healing treatments [1]. A synthetic polymer such as PLA is a biodegradable, biocompatible, and non-toxic polymer material that is commonly used in the tissue engineering field [1]. On the other hand, the natural polysaccharide Chi is gaining more attention due to its superior regenerative, antibacterial, and anti-inflammatory effects [2]. These characteristics of biomaterials allow their use in biomedical applications such as tissue engineering, drug delivery, and wound dressings [3].
A relatively ideal nanomatrix for wound healing applications should possess strong mechanical properties as well as appropriate chemical and biological properties that enable a non-toxicity medium for cell proliferation, adhesion and the ability to facilitate wound re-epethelization.
The focus of this study is on fabricating relatively ideal materials from a combination of natural and synthetic polymers, with the aim of accelerating the regeneration of damaged skin tissue.
2. Materials and Methods
Poly(lactic acid) (PLA) (average Mn ~ 60,000, 26100-51-6) and Chitosan (Chi) (medium molecular weight, 448,877) were purchased from Sigma-Aldrich. Acetic acid glacial (AcA) was purchased from Fisher Chemical (64-19-7). Dullbeco Modified Eagle Medium (DMEM) was obtained from Thermo Fisher, while Phosphate Buffered Solution (PBS) was obtained from Sigma Aldrich. In this study, all chemicals were used without further purification.
2.1. Methods
2.1.1. Preparation of Polymer Solution
The examined solution was prepared by dissolving the 19% Chi:PLA (volume ratio 1:1) in a combination of glacial acetic acid:H2O (volume ratio 1:1). The prepared sample was placed on magnetic stir at 750 rpm for 24 h. After the time had expired, the homogenous solution was loaded into a syringe with a 21 G needle. The applied flow rate was 0.8 mL/h−1. The duration of the process was 5 min for each run. The aluminum plate was used as a collector for the nanofibers.
2.1.2. Process of Electrospinning
The electrospinning technique is based on the application of a high electric potential between two electrodes with opposite polarities [4]. The standard setup consists of a high-voltage supply, a syringe with a needle, and a grounded fiber collector. The whole process was controlled via appropriate house-made computer software [5].
2.2. Cytotoxicity Assay
The cytocompatibility, proliferation, and viability of cells cultured on scaffolds were determined by MTT standardized protocol (Laboratory for Bioengineering protocol CB-005). MTT is a chemical compound (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) which represents a yellow tetrazolium water-soluble salt. The cell cytotoxicity assay is based on the reduction of tetrazolium salt in insoluble purple formazan products by the mitochondria of viable cells. As a result of the formazan crystals precipitating in viable cells, the color change indicates the percentage of viable and dead cells.
The scaffolds were placed under UV light for 2 h before they were cut into round specimens (5 mm × 5 mm). The prepared scaffolds were stuck in each bottom of a sterile 96-well plate with added DMEM for 24 h. The DMEM was then removed and the scaffolds were seeded with an MRC-5 cell line. The optimized density of seeded cells was 1 × 104 cells/100 µL on the surface of scaffolds in each well. For positive control, cells were seeded without scaffolds.
The plate was incubated at 37 °C and 5% CO2 for different incubation times (24, 72, and 120 h). Afterward, the scaffolds’ supernatant was removed and rinsed with 5 mL of PBS. The MTT solution (25 µL) was mixed with DMEM (100 µL) and added to each well at 37 °C for 4 h. After incubation time, the culture medium was removed and 150 µL DMSO was added. The reduction of the MTT compound by viable cells exerted the purple formazan crystals. The absorbance was measured at 490 nm on an ELISA Rayto reader. The obtained data were expressed as mean ± standard error.
2.3. In Vitro Wound Healing
A sterile 6-well plate was used to seed a healthy lung fibroblast cell line (MRC-5) with a cell suspension density of 1 × 104/well. Cells were incubated for 24 h at 37 °C and 5% CO2. After reaching 80–90% confluence, the scratch was implemented by using a 10 µL pipette tip to create the cell-free area. After scratching, the non-adherent cells were washed with PBS. The electrospun scaffolds were added to each culture well and kept at physiological conditions. Cell migration between scratch areas was observed with an optical microscope (Delta Optical Genetic PRO microscope, Poland) at 0 h, initially before, and 24 h after placed membranes. Image J software package (model) was used to measure the wound gap using the following Equation (1):
where W0 and Wd are the gaps between scratch and after 24 h of incubation of membranes, respectively.
3. Results
3.1. Cytotoxicity Assay
To investigate the potential biomedical applications of the produced nanofibers, the cytocompatibility of Chi:PLA nanomatrices was examined via MTT cell viability assay. The percentage of cell viability was presented as 96.02%, 111.88%, and 404.55% at 24, 72, and 120 h, respectively (Figure 1).
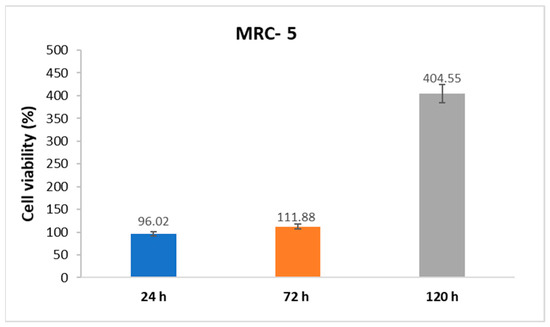
Figure 1.
MTT viability assay of cells proliferated onto Chi:PLA scaffolds.
3.2. In Vitro Wound Healing
Figure 2 shows the percentage of relative wound closure after treatment with Chi:PLA scaffolds. The percentage of control untreated closure after 24 h was 67.87%, while the percentage of treated closure with scaffold was 89.13%.
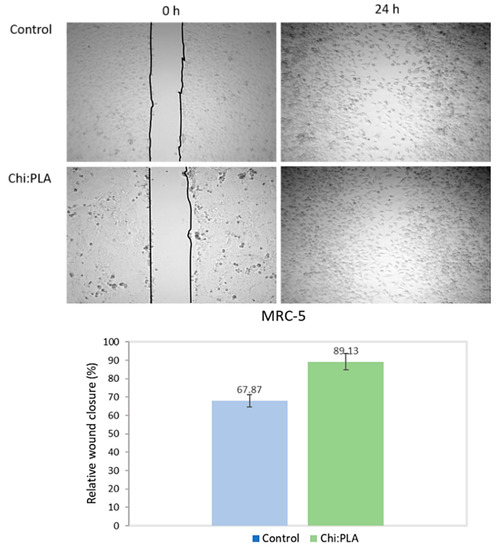
Figure 2.
In vitro wound healing in the treatment of Chi:PLA nanomatrices.
4. Discussion
4.1. Cytotoxicity Assay
The results in the MTT assay indicated that Chi:PLA scaffolds, i.e., nanomatrices, did not induce cytotoxic effects on healthy fibroblast cells. The viability of cells cultured onto Chi:PLA nanofiber mats was similar after 24 and 72 h. Furthermore, cell proliferation on used scaffolds increased with increasing incubation time, indicating that the nanofibers did not have cytotoxic effects on healthy fibroblasts. The proliferation of cells was significantly increased after 120 h (404.55%) in comparison with treatment after 24 h. This result suggests that the Chi:PLA scaffolds exhibited good biocompatibility, inducing increased cell viability and proliferation.
4.2. In Vitro Wound Healing
Cell migration is a crucial step for wound healing. According to the obtained results, the closure of wound in the treatment with Chi:PLA scaffolds after 24 h was 89.13%, while the value from the control data was 67.87% (Figure 2). The results of the scaffold treatment not only show no negative effect on migration, but also promote the migration of healthy fibroblasts to the wound gap. However, in the treatment of cytotoxicity, the scaffolds caused statistically significant proliferation after the fifth day. On that basis, it can be assumed that after a prolonged time of wound exposure to treatment with scaffolds, the scaffolds would promote a higher rate of proliferation and differentiation of the healthy cell culture in the treatment of wound healing.
5. Conclusions
In summary, the major challenge in tissue engineering is to fabricate a relatively ideal scaffold with a surface suited to cell attachment, differentiation, and proliferation. According to the results of this study, Chi:PLA nanofibers produced by the electrospinning technique have superior performance for biomedical applications. The non-toxicity and regenerative effects of used scaffolds indicate the success of the selected materials and the optimization of the process parameters. The produced scaffolds promote cell adhesion and their growth in in vitro conditions, which is crucial for wound healing. The results of our study suggest that Chi:PLA scaffolds are useful and prominent in the application of wound healing.
Author Contributions
Conceptualization, K.V., N.F. and M.Ž.; methodology, K.V., B.M. and M.J.; validation, D.N.; investigation, K.V. and B.M.; resources, N.F. and M.Ž.; writing—original draft preparation, K.V. and B.M.; visualization, B. M., N.K. and J.G. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for financial support from the Ministry of Education, Science, and Technological Development of the Republic of Serbia (Agreement No. 451-03-68/2022-14/200378).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of poly(lactic acid): A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Rodríguez, R.; Espinoza, H.; Carvajal, C. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Feltz, K.; Emily, A.; Kalaf, G.; Chen, C.; Martin, S.; Sell, S. Research Article A review of electrospinning manipulation techniques to direct fiber deposition and maximize pore size. Electrospinning 2017, 1, 46–61. [Google Scholar] [CrossRef]
- Virijević, K.; Grujić, J.; Kokanović, M.; Nikolić, D.; Živanović, M.; Filipović, N. Electrospinning and Electrospun Nanofibrous Materials–Promising Scaffolds in Tissue Engineering. In Proceedings of the International Conference on Medical and Biological Engineering—CMBEBIH, Mostar, Bosnia and Herzegovina, 21–24 April 2021; Volume 84, pp. 726–733. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).