Salt-Induced Recovery of Volatile Organic Acids Using Non-Ionic Surfactants †
Abstract
Featured Application
Abstract
1. Introduction
2. Mechanism of Salting-Out
3. Adjustment on the Cloud Point due to Electrolytes
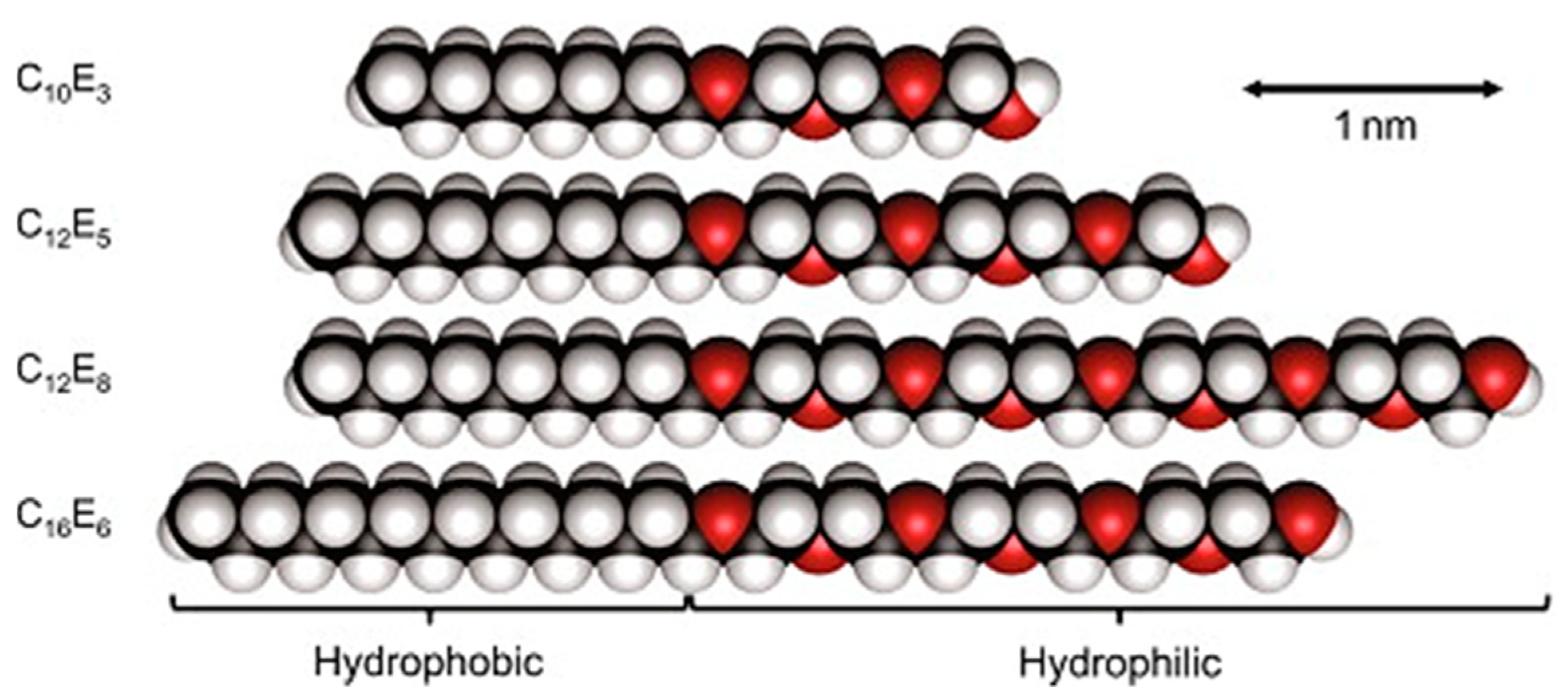
4. Relationship between Cloud Point and HLB
5. Response of Hydrophilic Units to Kosmotropicity
6. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reyhanitash, E.; Kersten, S.R.A.; Schuur, B. Recovery of volatile fatty Acids from fermented wastewater by adsorption. ACS Sustain. Chem. Eng. 2017, 5, 9176–9184. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-J.; Lim, S.-J.; Chang, H.N. Volatile fatty acid platform: concept and application. In Emerging Areas in Bioengineering; Stephanopoulos, G., Chang, H.N., Nielsen, J., Lee, S.Y., Eds.; Wiley-VCH Verlag GmbH & Co., KGaA: Darmstadt, Germany, 2017; Volume 1, pp. 173–190. [Google Scholar] [CrossRef]
- Baumann, I.; Westermann, P. Microbial production of short chain fatty acids from lignocellulosic biomass: current processes and market. BioMed Res. Int. 2016, 2016, 8468357. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, M.; Xu, M.; Yang, S.T. Anaerobic fermentation for production of carboxylic acids as bulk chemicals from renewable biomass. In Anaerobes in Biotechnology; Hatti-Kaul, R., Mamo, G., Mattiasson, B., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 323–361. [Google Scholar] [CrossRef]
- De Oliveira, R.A.; Komesu, A.; Rossell, C.E.V.; Filho, R.M. Challenges and opportunities in lactic acid bioprocess design—From economic to production aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- The Sources and Solutions: Wastewater. Available online: https://www.epa.gov/nutrientpollution/sources-and-solutions-wastewater#:~:text=Wastewater%20treatment%20facilities%20in%20the,gallons%20of%20wastewater%20every%20day (accessed on 10 October 2022).
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]
- Playne, M.J.; Smith, B.R. Toxicity of organic extraction reagents to anaerobic bacteria. Biotechnol. Bioeng. 1983, 25, 1251–1265. [Google Scholar] [CrossRef]
- Li, J.-L.; Chen, B.-H. Surfactant-mediated biodegradation of polycyclic aromatic hydrocarbons. Materials 2009, 2, 76–94. [Google Scholar] [CrossRef]
- Hyde, A.M.; Zultanski, S.L.; Waldman, J.H.; Zhong, Y.-L.; Shevlin, M.; Peng, F. General principles and strategies for salting-out informed by the hofmeister series. Org. Process. Res. Dev. 2017, 21, 1355–1370. [Google Scholar] [CrossRef]
- Randall, M.; Failey, C.F. The Activity Coefficient of non-electrolytes in aqueous salt solutions from solubility measurements. The Salting-out Order of the Ions. Chem. Rev. 1927, 4, 285–290. [Google Scholar] [CrossRef]
- Adsorption. Available online: https://www.surfactantassociates.com/resources/adsorption (accessed on 10 October 2022).
- Trawińska, A.; Hallmann, E.; Mędrzycka, K. Synergistic effects in micellization and surface tension reduction in nonionic gemini S-10 and cationic RTAB surfactants mixtures. Colloids Surf. A Physicochem. Eng. Asp. 2016, 488, 162–172. [Google Scholar] [CrossRef]
- Goswami, A.; Hassan, P.; Bhagwat, S.S. Static and dynamic surface tension behaviour of a triblock copolymer and a non-ionic surfactant mixture. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 190–196. [Google Scholar] [CrossRef]
- Fan, Y.; Tang, H.; Wang, Y. Synergistic behavior and microstructure transition in mixture of zwitterionic surfactant, anionic surfactant, and salts in sorbitol/H2O solvent: 1. Effect of Surfactant Compositions. J. Surfactants Deterg. 2017, 20, 435–443. [Google Scholar] [CrossRef]
- Ferreira, J.; Mikhailovskaya, A.; Chenneviere, A.; Restagno, F.; Cousin, F.; Muller, F.; Degrouard, J.; Salonen, A.; Marques, E.F. Interplay between bulk self-assembly, interfacial and foaming properties in a catanionic surfactant mixture of varying composition. Soft Matter 2017, 13, 7197–7206. [Google Scholar] [CrossRef] [PubMed]
- Fainerman, V.; Kovalchuk, V.; Aksenenko, E.; Ravera, F.; Liggieri, L.; Loglio, G.; Makievski, A.; Mishchuk, N.; Schneck, E.; Miller, R. A Multistate Adsorption Model for the Adsorption of C14EO4 and C14EO8 at the Solution/Air Interface. Colloids Interfaces 2021, 5, 39. [Google Scholar] [CrossRef]
- Fainerman, V.; Aksenenko, E.; Kovalchuk, V.; Mucic, N.; Javadi, A.; Liggieri, L.; Ravera, F.; Loglio, G.; Makievski, A.; Schneck, E.; et al. New view of the adsorption of surfactants at water/alkane interfaces—Competitive and cooperative effects of surfactant and alkane molecules. Adv. Colloid Interface Sci. 2020, 279, 102143. [Google Scholar] [CrossRef]
- Zhao, Y.; Qi, K.; Zhu, B.; Long, H.; Huang, Q.; Lei, F.; Huang, Z.; Zhou, J. Effect of chain rigidity on morphological transformation and adsorption behaviors of biocompatible polyoxyethylene-based surfactants at water–air surface. Colloid Polym. Sci. 2021, 299, 1807–1817. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O. Adsorption of ionic surfactants on water/air interface: one more transformation of the Gibbs equation. Surf. Eng. Appl. Electrochem. 2014, 50, 173–182. [Google Scholar] [CrossRef]
- Fainerman, V.B.; Miller, R.; Möhwald, H. General relationships of the adsorption behavior of surfactants at the water/air interface. J. Phys. Chem. B 2002, 106, 809–819. [Google Scholar] [CrossRef]
- Luan, H.; Gong, L.; Yue, X.; Nie, X.; Chen, Q.; Guan, D.; Que, T.; Liao, G.; Su, X.; Feng, Y. Micellar aggregation behavior of alkylaryl sulfonate surfactants for enhanced oil recovery. Molecules 2019, 24, 4325. [Google Scholar] [CrossRef]
- Aveyard, B. Aggregation of surfactants in aqueous systems. In Surfactants: In Solution, at Interfaces and in Colloidal Dispersions; Aveyard, B., Ed.; Oxford University Press: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Poole, C.F. Chapter 1—Milestones in the development of liquid-phase extraction Techniques. In Liquid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–44. [Google Scholar] [CrossRef]
- Anton, N. Salting-out effect induced by temperature cycling on a water/non-ionic surfactant/oil system. J. Phys. Chem. B 2007, 111, 3651–3657. [Google Scholar] [CrossRef]
- Amani, P.; Firouzi, M. Effect of divalent and monovalent salts on interfacial dilational rheology of sodium dodecylbenzene sulfonate solutions. Colloids Interfaces 2022, 6, 41. [Google Scholar] [CrossRef]
- Araújo, A.A.D.L.; Neto, E.L.D.B.; Filho, O.C.; Foletto, E.L. Federal University of Santa Maria. Influence of sodium chloride on the cloud point of polyethoxylate surfactants and estimation of Flory-Huggins model parameters. Rev. Fac. De Ing. Univ. De Antioq. 2015, 1, 155–162. [Google Scholar] [CrossRef]
- Myers, D. Surfactants in solution: monolayers and micelles. In Surfactant Science and Technology, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 107–159. [Google Scholar] [CrossRef]
- Al-Sabagh, A.; Nasser, N.; Migahed, M.; Kandil, N. Effect of chemical structure on the cloud point of some new non-ionic surfactants based on bisphenol in relation to their surface-active properties. Egypt. J. Pet. 2011, 20, 59–66. [Google Scholar] [CrossRef]
- Tasaki, K. Poly(oxyethylene)—water interactions: a molecular dynamics study. J. Am. Chem. Soc. 1996, 118, 8459–8469. [Google Scholar] [CrossRef]
- Shen, Y.-H. Cloud point foaming technique for separation of nonionic surfactant from solution. Sep. Sci. Technol. 1997, 32, 2229–2235. [Google Scholar] [CrossRef]
- Man, B.K.-W.; Lam, M.H.-W.; Lam, P.K.S.; Wu, R.S.S.; Shaw, G. Cloud-point extraction and preconcentration of cyanobacterial toxins (microcystins) from natural waters using a cationic surfactant. Environ. Sci. Technol. 2002, 36, 3985–3990. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.M.; Bernin, D.; Topgaard, D. NMR studies of nonionic surfactants. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 79, pp. 73–127. [Google Scholar] [CrossRef]
- Borges, F.T.P.; Papavasiliou, G.; Teymour, F. Synthesis of polyphosphate-loaded nanoparticles using inverse miniemulsion polymerization for sustained delivery to the gastrointestinal tract. Macromol. React. Eng. 2019, 13, 1800068. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, K.; Takeda, H. The effect of added salts in water on the hydrophile-lipophile balance of nonionic surfactants: The effect of added salts on the phase inversion temperature of emulsions. J. Colloid Interface Sci. 1970, 32, 642–646. [Google Scholar] [CrossRef]
- Marszall, L.; Van Valkenburg, J.W. The effect of glycols on the hydrophile-lipophile balance and the micelle formation of nonionic surfactants. J. Am. Oil Chem. Soc. 1982, 59, 84–87. [Google Scholar] [CrossRef]
- Marszall, L. The effective hydrophile-lipophile balance of nonionic surfactants. J. Jpn. Oil Chem. Soc. 1983, 32, 135–144. [Google Scholar] [CrossRef]
- Kothencz, R.; Nagy, R.; Bartha, L. Determination of HLB values of some nonionic surfactants and their mixtures. Stud. Univ. Babes-Bolyai. Chem. 2017, 62, 451–458. [Google Scholar] [CrossRef]
- Arkhipov, V.P.; Arkhipov, R.; Filippov, A. Micelles of oxyethylated isononylphenols in aqueous solutions and hydrophilic–lipophilic balance. ACS Omega 2020, 5, 28224–28232. [Google Scholar] [CrossRef]
- Nostro, P.L.; Ninham, B.W. Hofmeister phenomena: an update on ion specificity in biology. Chem. Rev. 2012, 112, 2286–2322. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kang, T.H.; Kim, M.W.; Han, S. Ion specific effects: Decoupling ion–ion and ion–water interactions. Phys. Chem. Chem. Phys. 2015, 1713, 8306–8322. [Google Scholar] [CrossRef] [PubMed]
- Naseem, B.; Arif, I.; Jamal, M.A. Kosmotropic and chaotropic behavior of hydrated ions in aqueous solutions in terms of expansibility and compressibility parameters. Arab. J. Chem. 2021, 14, 103405. [Google Scholar] [CrossRef]
- Ren, C.-L.; Tian, W.-D.; Szleifer, I.; Ma, Y.-Q. Specific salt effects on poly(ethylene oxide) electrolyte solutions. Macromolecules 2011, 44, 1719–1727. [Google Scholar] [CrossRef]
- Padmanabhan, V.; Daillant, J.; Belloni, L.; Mora, S.; Alba, M.; Konovalov, O. Specific ion adsorption and short-range interactions at the air aqueous solution interface. Phys. Rev. Lett. 2007, 99, 086105. [Google Scholar] [CrossRef]
- Ghosal, S.; Hemminger, J.C.; Bluhm, H.; Mun, B.S.; Hebenstreit, E.L.D.; Ketteler, G.; Ogletree, D.F.; Requejo, F.G.; Salmeron, M. Electron spectroscopy of aqueous solution interfaces reveals surface enhancement of halides. Science 2005, 307, 563–566. [Google Scholar] [CrossRef]
- Jungwirth, P.; Tobias, D.J. Molecular structure of salt solutions: a new view of the interface with implications for heterogeneous atmospheric chemistry. J. Phys. Chem. B 2001, 105, 10468–10472. [Google Scholar] [CrossRef]
- Sergeeva, V.F. Salting-out and salting-in of non-electrolytes. Russ. Chem. Rev. 1965, 34, 309–318. [Google Scholar] [CrossRef]
- Görgényi, M.; Dewulf, J.; Van Langenhove, H.; Héberger, K. Aqueous salting-out effect of inorganic cations and anions on non-electrolytes. Chemosphere 2006, 65, 802–810. [Google Scholar] [CrossRef]
- Wang, C.; Lei, Y.D.; Wania, F. Effect of sodium sulfate, ammonium chloride, ammonium nitrate, and salt mixtures on aqueous phase partitioning of organic compounds. Environ. Sci. Technol. 2016, 50, 12742–12749. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Sabatini, D.A. Separation of organic compounds from surfactant solutions: A Review. Sep. Sci. Technol. 2007, 42, 453–475. [Google Scholar] [CrossRef]
- Jiang, C.; Ma, J. De-Inking of Waste Paper: Flotation. Encycl. Sep. Sci. 2000, 2537–2544. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Dou, H.; Pei, L. Influence of ethylene oxide content in nonionic surfactant to the hydrolysis of reactive dye in silicone non-Aqueous dyeing system. Polymers 2018, 10, 1158. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Mandal, A.; Belhaj, H.; Kumar, T. Enhanced oil recovery by nonionic surfactants considering micellization, surface, and foaming properties. Pet. Sci. 2017, 14, 362–371. [Google Scholar] [CrossRef]
- Esmaeili, H.; Mousavi, S.M.; Hashemi, S.A.; Lai, C.W.; Chiang, W.-H.; Bahrani, S. Application of biosurfactants in the removal of oil from emulsion. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Smirnova, A., Numan-Al-Mobin, A., Inamuddin, Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 107–127. [Google Scholar] [CrossRef]
- Kapse, A.; Anup, N.; Patel, V.; Saraogi, G.K.; Mishra, D.K.; Tekade, R.K. Chapter 6 - Polymeric micelles: A ray of hope among new drug delivery systems. In Drug Delivery Systems; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 235–289. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatdula, K.M.; Revellame, E.D. Salt-Induced Recovery of Volatile Organic Acids Using Non-Ionic Surfactants. Eng. Proc. 2023, 31, 20. https://doi.org/10.3390/ASEC2022-13817
Gatdula KM, Revellame ED. Salt-Induced Recovery of Volatile Organic Acids Using Non-Ionic Surfactants. Engineering Proceedings. 2023; 31(1):20. https://doi.org/10.3390/ASEC2022-13817
Chicago/Turabian StyleGatdula, Kristel M., and Emmanuel D. Revellame. 2023. "Salt-Induced Recovery of Volatile Organic Acids Using Non-Ionic Surfactants" Engineering Proceedings 31, no. 1: 20. https://doi.org/10.3390/ASEC2022-13817
APA StyleGatdula, K. M., & Revellame, E. D. (2023). Salt-Induced Recovery of Volatile Organic Acids Using Non-Ionic Surfactants. Engineering Proceedings, 31(1), 20. https://doi.org/10.3390/ASEC2022-13817





